Global Adalimumab Biosimilar Market by Indication (Rheumatoid Arthritis, Juvenile Idiopathic Arthritis, Psoriatic Arthritis, Other Indications), By Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Other Distribution Channels), and by Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2022-2032
- Published date: Aug 2022
- Report ID: 36720
- Number of Pages: 274
- Format:
- keyboard_arrow_up
Adalimumab Biosimilar Market Overview:
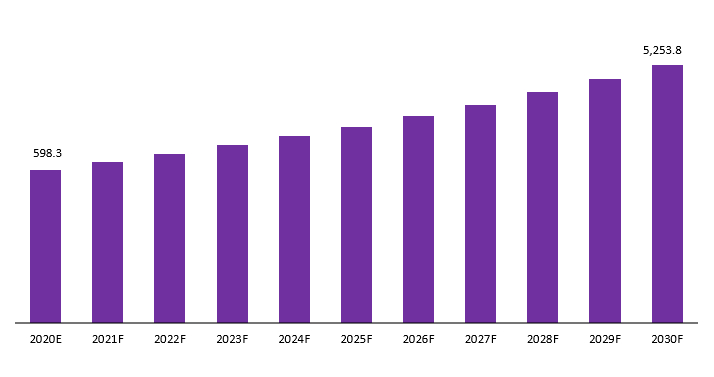
The global adalimumab biosimilar market is projected to be USD 598.3 Mn in 2022 to reach USD 5,253.8 Mn by 2032 at a CAGR of 23.9%.
Adalimumab is a fully-humanized monoclonal antibody that was approved in 2002 by the Food and Drug Administration (FDA). Adalimumab, sold under the brand name ‘Humira’, is the bestselling drug in terms of revenue share in the overall market worldwide.
When Humira’s patent expired in 2016, companies including Novartis, Mylan, Biogen, and Amgen immediately began selling biosimilar versions of the parent drug.
A biosimilar product is a biological agent that is considered highly similar to an approved biological drug, which is known as the reference product. Biological products are derived from a living organism, including sources such as humans, animals, microorganisms, or yeast.
A biosimilar is a similar version of original biological medicine. A biosimilar product needs to adhere to specific guidelines mandated by a regulatory health authority, such as the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) regulatory.
To be approved as a biosimilar for a particular reference biologic, the product must be similar to the reference product and have no differences in terms of safety or efficacy, and should be approved for the indication(s) and condition(s) for which the reference product has been approved, should be in the same Indication as well as dosage as the reference product, must have the same mechanism of action as the reference product.
Adalimumab biosimilars are effective and equally safe. All the biosimilar versions of adalimumab are generally used to treat inflammation of the skin (hidradenitis suppurativa and plaque psoriasis), joints (polyarticular juvenile idiopathic arthritis, rheumatoid arthritis, and active enthesitis-related arthritis), joints and skin (psoriatic arthritis), etc. Adalimumab biosimilars medicines are available by prescription.
Global Adalimumab Biosimilar Market Revenue (USD Mn), 2020–2030:

The rising incidence of autoimmune diseases such as irritable bowel syndrome, psoriasis, colitis, arthritis, kidney conditions, Crohn’s disease, cancers, and many others is a significant factor driving the growth of the global adalimumab biosimilar market.
However, the high cost associated with the development of adalimumab biosimilar is a factor anticipated to restrain the growth of the global adalimumab biosimilar market.
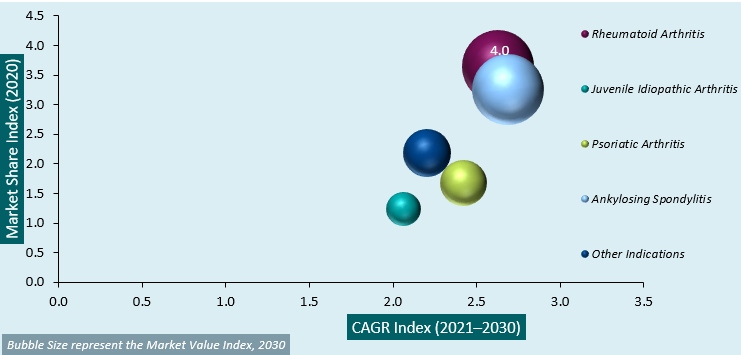
Global Adalimumab Biosimilar Market Attractiveness Analysis by Indication, 2014–2030:

The global adalimumab biosimilar market is segmented based on indication, distribution channel, and regions. Among the indication segments, the rheumatoid arthritis segment accounts for a high revenue share due to the rising prevalence rate of the disorder and extensive use of biosimilar for the treatment of a disorder.
Based on the distribution channel, the hospital pharmacies segment accounts for a significant share in terms of revenue. This is primarily attributed to a majority of procedures being perIndicationed at hospitals.
The market in Europe is expected to account for the majority revenue share for 2020 and is projected to dominate the global adalimumab biosimilar market over the forecast period. This projection can be attributed to the launch of several biosimilars in the region.
North America is expected to show remarkable growth after the launch of biosimilars in 2023 by major manufacturers.

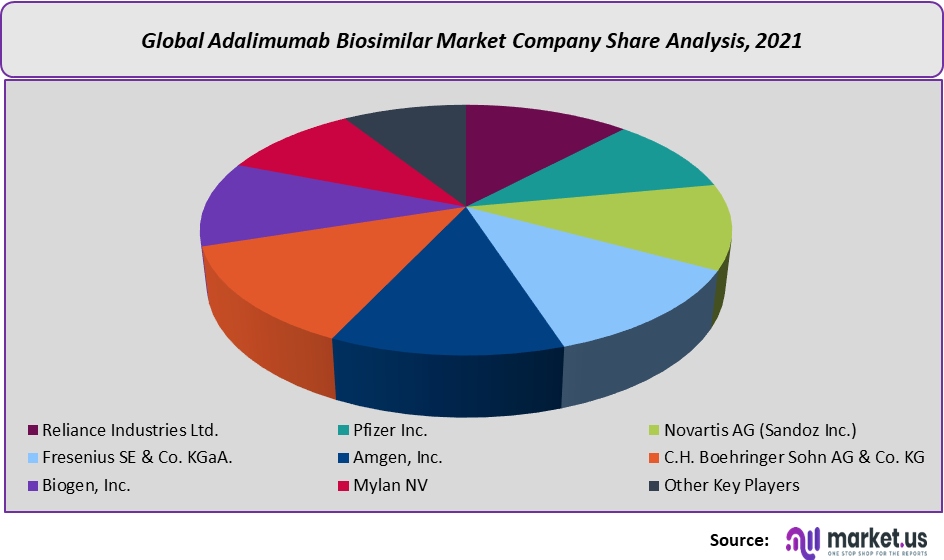
The research report on the global adalimumab biosimilar market includes profiles of some of the major companies such as:
- Reliance Industries Ltd.
- Pfizer Inc.
- Novartis AG (Sandoz Inc.)
- Fresenius SE & Co. KGaA.
- Amgen, Inc.
- C.H. Boehringer Sohn AG & Co. KG
- Biogen, Inc.
- Mylan NV
- Glenmark Pharmaceuticals Ltd.
- Torrent Pharmaceuticals Ltd.
- Samsung Bioepis
- Zydus Cadila Healthcare Limited
- Hetero Drugs
- Bio-Thera Solutions Ltd.
- Others.
Segmentation of Global Adalimumab Biosimilar Market is Indication, Distribution Channel, and Region:
Based on Indication:
• Rheumatoid Arthritis
• Juvenile Idiopathic Arthritis
• Psoriatic Arthritis
• Ankylosing Spondylitis
• Other IndicationsBased on Distribution Channel:
• Hospital Pharmacy
• Retail Pharmacy
• Online Pharmacy
• Other Distribution ChannelsBased on Region:
• North America
• Europe
• Asia Pacific
• South America
• Middle East & AfricaFor the Adalimumab Biosimilar Market research study, the following years have been considered to estimate the market size:
Attribute Report Details Historical Years
2016-2020
Base Year
2021
Estimated Year
2022
Short Term Projection Year
2028
Projected Year
2023
Long Term Projection Year
2032
Report Coverage
Competitive Landscape, Revenue analysis, Company Share Analysis, Manufacturers Analysis, Volume by Manufacturers, Key Segments, Key company analysis, Market Trends, Distribution Channel, Market Dynamics, COVID-19 Impact Analysis, strategy for existing players to grab maximum market share, and more.
Regional Scope
North America, Europe, Asia-Pacific, South America, Middle East & Africa
Country Scope
United States, Canada and Mexico, Germany, France, UK, Russia and Italy, China, Japan, Korea, India and Southeast Asia, Brazil, Argentina, Colombia etc.Saudi Arabia, UAE, Egypt, Nigeria and South Africa
![Adalimumab Biosimilar Market Adalimumab Biosimilar Market]() Adalimumab Biosimilar MarketPublished date: Aug 2022add_shopping_cartBuy Now get_appDownload Sample
Adalimumab Biosimilar MarketPublished date: Aug 2022add_shopping_cartBuy Now get_appDownload Sample - Reliance Industries Ltd.
- Pfizer Inc Company Profile
- Novartis AG (Sandoz Inc.)
- Fresenius SE & Co. KGaA.
- Amgen, Inc.
- C.H. Boehringer Sohn AG & Co. KG
- Biogen, Inc.
- Mylan NV
- Glenmark Pharmaceuticals Ltd.
- Torrent Pharmaceuticals Ltd.
- Samsung Bioepis
- Zydus Cadila Healthcare Limited
- Hetero Drugs
- Bio-Thera Solutions Ltd.
- Others.
- settingsSettings
Our Clients
|
Single User
$5,999
$2,999
USD / per unit
save 50% |
Multi User
$7,999
$3,499
USD / per unit
save 55% |
Corporate User
$12,999
$4,499
USD / per unit
save 65% | |
|---|---|---|---|
| e-Access | |||
| Data Set (Excel) | |||
| Company Profile Library Access | |||
| Interactive Dashboard | |||
| Free Custumization | No | up to 10 hrs work | up to 30 hrs work |
| Accessibility | 1 User | 2-5 User | Unlimited |
| Analyst Support | up to 20 hrs | up to 40 hrs | up to 50 hrs |
| Benefit | Up to 20% off on next purchase | Up to 25% off on next purchase | Up to 30% off on next purchase |
| Buy Now ($ 2,999) | Buy Now ($ 3,499) | Buy Now ($ 4,499) |