Global Cardiac Biomarkers Market, by Product Type (Myocardial Muscle Creatine Kinase (CK-MB), Troponins (T and I), Myoglobulin, Brain Natriuretic Peptide (BNPs) or NT-proBNP, Ischemia-modified Albumin (IMA), Other Product Types) By Location of Testing (Laboratory Testing, Point-of-Care Testing), and by Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2022-2032
- Published date: Feb 2022
- Report ID: 84380
- Number of Pages: 232
- Format:
- keyboard_arrow_up
Cardiac Biomarkers Маrkеt Overview
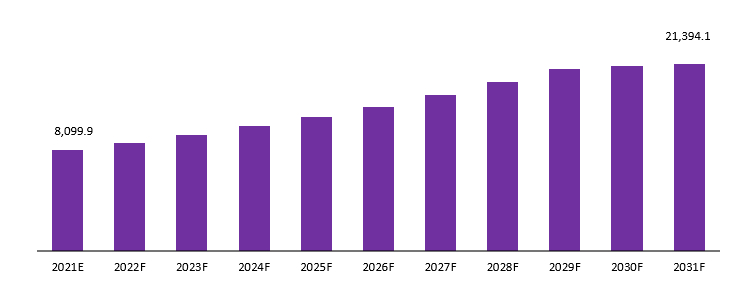
The global cardiac biomarkers market is projected to reach a valuation of USD 21,394.1 Mn by 2031 at a CAGR of 10.20%, from USD 8,099.9 Mn in 2021
Global Cardiac Biomarkers Market, by Product Type (Myocardial Muscle Creatine Kinase (CK-MB), Troponins (T and I), Myoglobulin, Brain Natriuretic Peptide (BNPs) or NT-proBNP, Ischemia-modified Albumin (IMA), Other Product Types) by Application (Myocardial Infarction, Congestive Heart Failure, Acute Coronary Syndrome, Atherosclerosis, Other Applications), By Location of Testing (Laboratory Testing, Point-of-Care Testing), and by Region – Global Forecast to 2031. This report offers a holistic view of the global cardiac biomarkers market through systematic segmentation that covers every aspect of this target market.
Cardiac biomarkers are a set of blood test measurements that can be used to screen for coronary artery disease and heart failure. They are medically beneficial in identifying cardiac diseases early when treatment is more likely to be successful. Cardiac biomarkers can detect a heart attack as soon as 30 minutes after it occurs. Additionally, cardiac biomarkers can screen for conditions like atrial fibrillation, myocardial infarction, and more. Cardiac biomarkers are among the best predictors of cardiovascular events. Cardiac biomarkers can also help identify problems that might not appear on an echocardiogram or electrocardiogram. The cardiac biomarkers troponin, and NT-proBNP, are substances found in the blood that can help diagnose cardiac disease. Troponin is a protein that causes muscle contraction. It is in higher quantities in the body when damage to skeletal and heart muscles (including heart attack). Still, it also can occur after strenuous exercise or even pregnancy. Cardiac biomarkers can also be detected during other medical conditions such as decompensated congestive heart failure and severe valvular disease. Biomarkers give medical professionals an insight into an individual’s health, especially those with chronic illnesses. Cardiac biomarkers are much more sensitive than ECGs when detecting cardiac injury. The use of cardiac biomarkers can help prevent sudden death in patients with presumed myocarditis.

Cardiac biomarkers are used to identify heart diseases by looking for substances found in the blood and can provide information about a person’s condition. This process of identifying a disease is called a biomarker test. One of these tests is an Electrocardiogram (EKG), which looks for anomalies that may show up in the electrical system of the heart.
Cardiac biomarkers are chemicals that can be detected in the blood or urine of heart patients and serve as an indicator of disease severity. These chemical levels are typically measured to establish a baseline before starting treatment and then periodically after treatment begins. It is important to note that these biomarkers may not always indicate heart disease. Clinicians should use caution when interpreting cardiac biomarkers since they may be elevated in other conditions such as systemic lupus erythematosus.
Global Cardiac Biomarkers Market Revenue (US$ Mn), 2021–2031

Source: Prudour, 2021
A host of variables influences the market growth of the cardiac biomarkers industry. The rise in demand for diagnostic and testing of various cardiovascular diseases, the discovery and progress of cardiac biomarkers, and development in the patient pool suffering from cardiovascular diseases are a few major drivers. Other important growth drivers include an increase in the number of new cardiovascular cases diagnosed each year and a surge in demand for disease-specific treatments that include cardiac biomarkers. According to the World Health Organization, around 17.7 million people die each year from Cardiovascular Diseases (CVDs), which account for 31% of all fatalities globally. Heart attacks and strokes account for over 80% of all CVD mortality. The ability of cardiac markers to detect heart failure accurately and quickly after the onset of chest discomfort has boosted industry expansion. Furthermore, promising aspects such as high accuracy, quick results, and cost-effective pricing of cardiac Point-of-Care (POC) testing propel the global growth of the cardiac biomarker market.
The market for cardiac biomarkers is being potentially restrained by inadequate specificity in certain circumstances and adverse effects such as skeletal muscle injury. Furthermore, the US Food and Drug Administration’s (FDA) rigorous validation and technological challenges linked to sample collecting and storage operations as market restraints. Companies can request regulatory certification of a biomarker for a specific context of usage in drug development through the FDA’s Biomarker Qualification Program. Only qualified biomarkers can be employed in numerous drug development programs without the need for the Center for Drug Evaluation and Research (CDER) to confirm the biomarkers’ eligibility, which stifles the further expansion of the cardiac biomarkers market.
The demand for research initiatives to increase the effectiveness of cardiac biomarker-based testing is growing. As a result, the market for cardiac biomarkers is expected to develop faster over the forecast period due to increased R&D activities. This market’s significant possibilities are the commercial deployment of multi-menu options for cardiac testing, employing various cardiac biomarkers, and target-oriented solutions. For instance, in 2019, Ortho Clinical Diagnostics, a U.S.-based in-vitro diagnostics company, developed a more rapid and accurate assay to detect a heart attack. This newly developed Vitros High Sensitivity Troponin I Assay is based on the quantitative measurement of troponin, a cardiac biomarker. These factors are expected to create lucrative opportunities for existing and emerging market players.
The Global Cardiac Biomarkers Market Segmentation
Based on Product Type, Application, Location of Testing, and Region
Based on Dosage Form
- Myocardial Muscle Creatine Kinase (CK-MB)
- Troponins (T and I)
- Myoglobulin
- Brain Natriuretic Peptide (BNPs) or NT-proBNP
- Ischemia-Modified Albumin (IMA)
- Other Product Types
Based on Application
- Myocardial Infarction
- Congestive Heart Failure
- Acute Coronary Syndrome
- Atherosclerosis
- Other Applications
Based on Location of Testing
- Laboratory Testing
- Point-of-Care Testing
Based on Region
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
This research report on the global cardiac biomarkers market includes major company profiles such as
- Abbott Laboratories
- F. Hoffmann-La Roche AG
- Thermo Fisher Scientific Inc.
- Bio-Rad Laboratories Inc.
- Danaher Corporation
- Randox Laboratories Ltd.
- Siemens Healthineers
- BioMérieux SA. and among others.
For the cardiac biomarkers market research study, the following years have been considered to estimate the market size:
Attribute Report Details Historical Years
2016-2020
Base Year
2021
Estimated Year
2022
Short Term Projection Year
2028
Projected Year
2023
Long Term Projection Year
2032
Report Coverage
Competitive Landscape, Revenue analysis, Company Share Analysis, Manufacturers Analysis, Volume by Manufacturers, Key Segments, Key company analysis, Market Trends, Distribution Channel, Market Dynamics, COVID-19 Impact Analysis, strategy for existing players to grab maximum market share, and more.
Regional Scope
North America, Europe, Asia-Pacific, South America, Middle East & Africa
Country Scope
United States, Canada and Mexico, Germany, France, UK, Russia and Italy, China, Japan, Korea, India and Southeast Asia, Brazil, Argentina, Colombia etc.Saudi Arabia, UAE, Egypt, Nigeria and South Africa
![Cardiac Biomarkers Маrkеt Cardiac Biomarkers Маrkеt]()
- Abbott Laboratories
- F. Hoffmann-La Roche AG
- Thermo Fisher Scientific Company Profile
- Bio-Rad Laboratories Inc.
- Danaher Corporation Company Profile
- Randox Laboratories Ltd.
- Siemens Healthineers
- BioMérieux SA. and among others.
- settingsSettings
Our Clients
|
Single User
$5,999
$2,999
USD / per unit
save 50% |
Multi User
$7,999
$3,499
USD / per unit
save 55% |
Corporate User
$12,999
$4,499
USD / per unit
save 65% | |
|---|---|---|---|
| e-Access | |||
| Data Set (Excel) | |||
| Company Profile Library Access | |||
| Interactive Dashboard | |||
| Free Custumization | No | up to 10 hrs work | up to 30 hrs work |
| Accessibility | 1 User | 2-5 User | Unlimited |
| Analyst Support | up to 20 hrs | up to 40 hrs | up to 50 hrs |
| Benefit | Up to 20% off on next purchase | Up to 25% off on next purchase | Up to 30% off on next purchase |
| Buy Now ($ 2,999) | Buy Now ($ 3,499) | Buy Now ($ 4,499) |