Global Clinical Biomarker Testing Market, by Disease Types (Cancer, Metabolism, Infectious Disease, Cardiology, Neurology) by Applications (Drug Discovery, Nutrigenomics, Toxicology Testing), By End-Users (Pharma and Biotech Companies, Diagnostic Tool Companies, Healthcare IT/Big Data Companies) and by Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2022-2032
- Published date: Feb 2022
- Report ID: 84784
- Number of Pages: 304
- Format:
- keyboard_arrow_up
Clinical Biomarker Testing Маrkеt Overview:
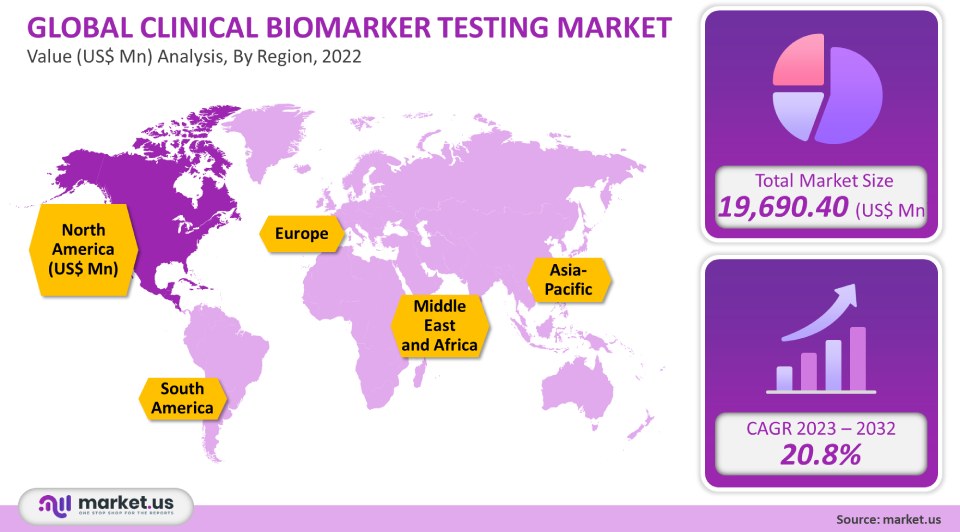
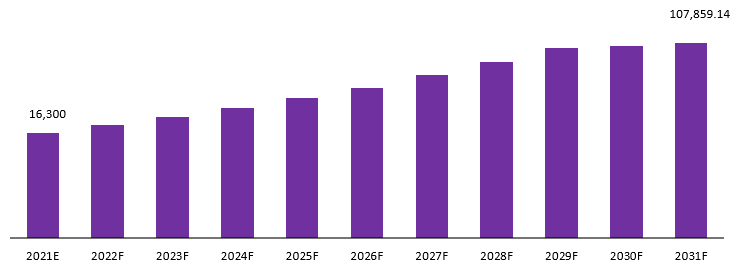
The global clinical biomarker testing market is projected to reach a valuation of USD 1,30,293.84 Mn by 2032 at a CAGR of 20.8%, from USD 19,690.40 Mn in 2022.
Clinical biomarker testing is a lab test that evaluates if blood protein levels are either too low, too high, or in a normal range. Biomarkers can be used to diagnose and monitor patients with diseases such as cancer and heart disease. The tests measures substances in the blood that may be elevated or decreased when an illness has been detected. Clinical biomarker testing is often used to help diagnose diseases. More than just identifying the presence of a condition, biomarker tests can also provide information on the severity and progression of a disease.

They are most often utilized in addition to other diagnostic techniques, such as imaging studies or lab work, but can be useful when these methods are not available or do not clearly indicate a definitive result. Clinical biomarkers are patient-specific markers of a disease, which may be more accurate than symptoms, and can provide more reliable information on the course of a disease. Tests for these biomarkers are very sensitive and specific compared to tests traditionally used to diagnose a condition, and can also give information on the prognosis or response to therapy. Clinical biomarkers may be more reliable as they do not depend on the subjective interpretation of symptoms by a patient.
Clinical biomarkers, which is a screening process, help to identify certain diseases or disorders. Biomarkers are often used to measure the severity of an illness or condition. For example, blood sugar levels can be tested as a biomarker for diabetes. In this scenario, people with higher levels of blood sugar would be more likely to have Type-2 diabetes than those individuals who maintained normal glucose levels. Clinical biomarkers are essential to understanding the inner workings of disease. Biomarkers are measurable substances in the body that can provide valuable information about the health of a person.
Biomarker testing is used to help physicians diagnose diseases in patients. It is most often utilized in clinical research studies where it is used to determine which treatments are more effective. Biomarkers are chemical substances found at certain levels in the body that indicate a physiological response. These tests are non-invasive, unlike other types of medical testing, and are easy for patients to provide samples for. Clinical biomarkers are a new area of medical sciences that promises to revolutionize how diseases are diagnosed and treated. Proteomics, a promising technique, that involves measuring proteins that are indicative of what is happening in the body, has already been used to successfully diagnose diseases such as HIV/AIDS, Alzheimer’s disease, and even cancer.
The use of biomarkers for clinical purposes is a relatively new scientific endeavor. This technique offers many advantages in diagnosis and treatment planning, such as providing information about the progression of a disease and the response to therapy. However, this technique also has certain disadvantages. The costs associated with these tests may be prohibitive for both doctors and patients. The process can take a long time and may delay an accurate diagnosis or treatment plan.
Global Clinical Biomarker Testing Market Revenue (USD Mn), 2021–2031:

The market for clinical biomarker testing has been designed to help healthcare providers cater to the needs of their patients. There is a need for the services of this market because clinicians typically rely on different types of data from different sources when they make a diagnosis, and often have to take samples from different areas of the body. Thus, clinicians can now use these tests to quickly determine whether they are dealing with cancerous cells or bacterial infections. Moreover, the rapid aging of global populations, combined with surging obesity rates, are elements that are expected to continue to drive the further expansion of the biomarker testing industry. Other factors contributing to the augmentation of this industry, in terms of revenue, include the increasing number of cancer medicines as well as improvements in bioinformatics.
The demand for clinical biomarkers is expected to increase in the future due to a spike in the number of individuals with diabetes. The clinical biomarker testing market is witnessing increasing interest by pharmaceutical companies in the field of biomarker-based drug development.
The demand for tailored pharmaceuticals in Europe and the United States is increasing, in turn propelling the clinical biomarker testing market forward. Furthermore, the demand for clinical biomarker testing in Europe and North America is being also driven by a growing need for toxicological technologies as well as considerable R&D efforts. As a result, Europe and North America accounted for the highest market share in 2019. On the other hand, markets in the Asia-Pacific region, pose immense growth potential on account of the growing number of clinical laboratories in countries like India and China.
 The high costs associated with cancer biomarker testing is a factor that is currently restraining this market’s revenue growth trajectory.
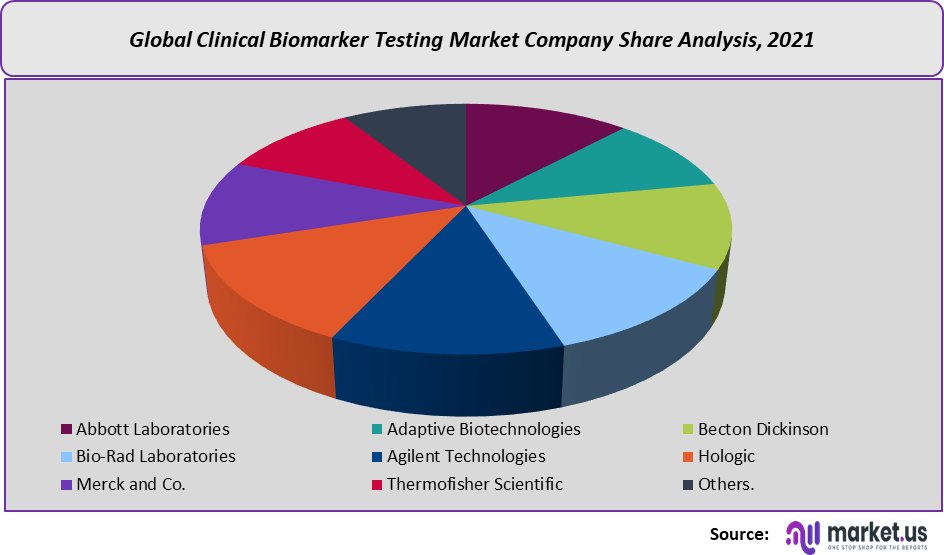
The high costs associated with cancer biomarker testing is a factor that is currently restraining this market’s revenue growth trajectory.This research report on the global clinical biomarker testing market includes major company profiles such:
- Abbott Laboratories
- Adaptive Biotechnologies
- Becton Dickinson
- Bio-Rad Laboratories
- Agilent Technologies
- Hologic
- Merck and Co.
- Thermofisher Scientific
- Danaher Corporation
- AbbVie Inc.
- Others.
The Global Clinical Biomarker Testing Market Segmentation is Based on Disease Types, Applications, End-Users, and Region
Based on Disease Types
- Cancer
- Metabolism
- Infectious Disease
- Cardiology
- Neurology
- Immunological Disease
Based on Applications
- Drug Discovery
- Nutrigenomics
- Toxicology Testing
- Personalized Medicine
- Functional Genomics
Based on End-Users
- Pharma and Biotech Companies
- Diagnostic Tool Companies
- Healthcare IT/Big Data Companies
- Clinical Laboratories
- Research Institutes
Based on Region
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
For the Clinical Biomarker Testing Market research study, the following years have been considered to estimate the market size:
Attribute Report Details Historical Years
2016-2020
Base Year
2021
Estimated Year
2022
Short Term Projection Year
2028
Projected Year
2023
Long Term Projection Year
2032
Report Coverage
Competitive Landscape, Revenue analysis, Company Share Analysis, Manufacturers Analysis, Volume by Manufacturers, Key Segments, Key company analysis, Market Trends, Distribution Channel, Market Dynamics, COVID-19 Impact Analysis, strategy for existing players to grab maximum market share, and more.
Regional Scope
North America, Europe, Asia-Pacific, South America, Middle East & Africa
Country Scope
United States, Canada and Mexico, Germany, France, UK, Russia and Italy, China, Japan, Korea, India and Southeast Asia, Brazil, Argentina, Colombia etc.Saudi Arabia, UAE, Egypt, Nigeria and South Africa
![Clinical Biomarker Testing Маrkеt Clinical Biomarker Testing Маrkеt]() Clinical Biomarker Testing МаrkеtPublished date: Feb 2022add_shopping_cartBuy Now get_appDownload Sample
Clinical Biomarker Testing МаrkеtPublished date: Feb 2022add_shopping_cartBuy Now get_appDownload Sample - Abbott Laboratories
- Adaptive Biotechnologies
- Becton Dickinson
- Bio-Rad Laboratories, Inc. Company Profile
- Agilent Technologies Inc. Company Profile
- Hologic
- Merck and Co.
- Thermofisher Scientific
- Danaher Corporation Company Profile
- AbbVie Inc. Company Profile
- Others.
- settingsSettings
Our Clients
|
Single User
$5,999
$2,999
USD / per unit
save 50% |
Multi User
$7,999
$3,499
USD / per unit
save 55% |
Corporate User
$12,999
$4,499
USD / per unit
save 65% | |
|---|---|---|---|
| e-Access | |||
| Data Set (Excel) | |||
| Company Profile Library Access | |||
| Interactive Dashboard | |||
| Free Custumization | No | up to 10 hrs work | up to 30 hrs work |
| Accessibility | 1 User | 2-5 User | Unlimited |
| Analyst Support | up to 20 hrs | up to 40 hrs | up to 50 hrs |
| Benefit | Up to 20% off on next purchase | Up to 25% off on next purchase | Up to 30% off on next purchase |
| Buy Now ($ 2,999) | Buy Now ($ 3,499) | Buy Now ($ 4,499) |


 The high costs associated with cancer biomarker testing is a factor that is currently restraining this market’s revenue growth trajectory.
The high costs associated with cancer biomarker testing is a factor that is currently restraining this market’s revenue growth trajectory.



