Global Clinical Trial Imaging Market Based on Product and Service (Services, Software), By Based on Modality (Ultrasound, X-ray, Echocardiography), By Application (Pharmaceutical Companies, Biotechnology Companies, Medical Device Manufacturer) By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2022-2032
- Published date: Feb 2022
- Report ID: 77788
- Number of Pages: 278
- Format:
- keyboard_arrow_up
Clinical Trial Imaging Market Overview:
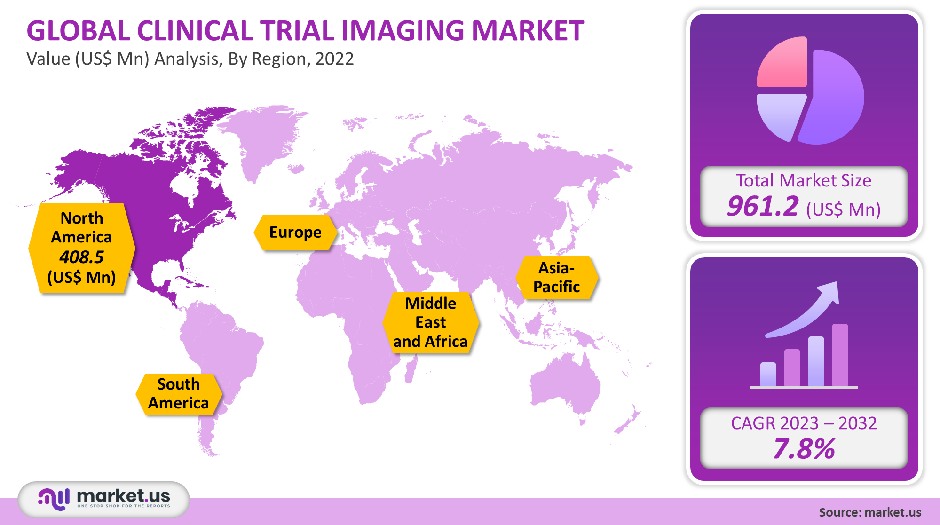
The market for clinical trial imaging was worth USD 961.2 million in 2021. This market is expected to grow at a CAGR of 7.8% between 2023-2032.
Market growth is expected to be driven by the increasing biotechnology and pharmaceutical industry, and increased research-and-development investments for new drugs to treat disease.
In the clinical development and commercialization of novel life-science products, medical imaging plays an important role.
Although the industry of medical imaging is constantly changing, the Biotechnology and Pharmaceutical industries continue to grow. This can be attributed mainly to increased investment in medical image companies and mergers & acquisitions. Also, innovative imaging technologies have been adopted to support clinical trials of medical devices.
Global Clinical Trial Imaging Market Scope:
Product and Service analysis
The market is segmented into service and software. The service segment accounts for 69.5% of revenue in the global share. The market also had a large share of operational imaging services. Operational imaging services include imaging modalities like MRI and CT. They also include ultrasound, OCT, and PET for therapeutic applications such as neurology and oncology.
Clinical trials often use imaging techniques to support decision-making. The 1997 Food and Drug Administration Modernization Act, (FDAMA), allowed imaging modalities as a tool for product development in medical devices and pharmaceutical clinical trials. Data generated by imaging modalities can be included in regulatory submissions.

End-User analysis
In 2021, the largest market share was held by Contract Research Organizations (CROs), which accounted for 46.2%. The significant market share can also be attributed to the rising cost of drug development and the increase in Research & Development activities.
The market is also growing due to the increasing demand from pharmaceutical and biotech companies for outsourcing research and development activities in order to cut costs. Contract research outsourcing collaborations provide cutting-edge services. Government organizations prefer to hand over projects to CROs.
Over the forecasted time, the biotechnology and pharmaceutical sector will be a rapidly growing segment. This segment’s rapid growth is due to the need for new treatments and drugs to treat chronic diseases. As competition is increasing, it is becoming more important for manufacturers to offer the best medicine/drug to end-users.
Biotechnology and pharmaceutical companies are making many innovative drug discoveries. The demand for clinical trial imaging will increase, which is expected to fuel the market growth.
Кеу Маrkеt Ѕеgmеntѕ
By Product and Service
- Ѕеrvісеѕ
- Ореrаtіоnаl Іmаgіng Ѕеrvісеѕ
- Rеаd Аnаlуѕіѕ Ѕеrvісеѕ
- Ѕуѕtеm and Тесhnісаl Ѕuрроrt Ѕеrvісеѕ
- Тrіаl Dеѕіgn & Соnѕultіng Ѕеrvісеѕ
- Ѕоftwаrе
By End-User
- Рhаrmасеutісаl Соmраnіеѕ
- Віоtесhnоlоgу Соmраnіеѕ
- Меdісаl Dеvісе Маnufасturеr
- Соntrасt Rеѕеаrсh Оrgаnіzаtіоnѕ
Market Dynamics:
The spread of COVID-19 in many countries has had a negative impact on the healthcare system. This has caused a disruption in research activities and medical studies. There have also been fewer sponsorships for clinical trial research. Many ongoing studies were delayed or canceled due to the pandemic. This has also hampered clinical trial timelines.
Market conditions have been adversely affected by unfavorable regulatory and guideline changes, supply chain disruptions, recruitment difficulties for clinical trials, fear of viral spread, and the closing down of most manufacturing plants during the lockdown. The introduction of virtual imaging trials in the COVID-19 pandemic will open up new opportunities for their adoption. Advanced computational models have allowed for a better assessment of CT images and radiography images. This will aid in the early diagnosis of COVID-19 among patients.
The increased use of imaging technology and the improved power of computing are expected to increase the use of imaging in clinical trials. Quantitative Imaging biomarkers Alliance protocol (QIBA), has developed standardized imaging protocols and methods that can be used to achieve precise and statistical endpoints in clinical trials.
The market growth may be limited by the high costs of equipment and their installation, as well as the huge cost of clinical trials. Technology advancements have made it possible to collect, evaluate, and submit clinical trial imaging data in a significant way.
Image analysis software and technology-enabled imaging offer many benefits for clinical trials, including consistency, data accuracy, adaptability, as well as compliance. Image analysis software, for example, is used to manage and direct a reader through the analysis of imaging time points.
Regional Analysis
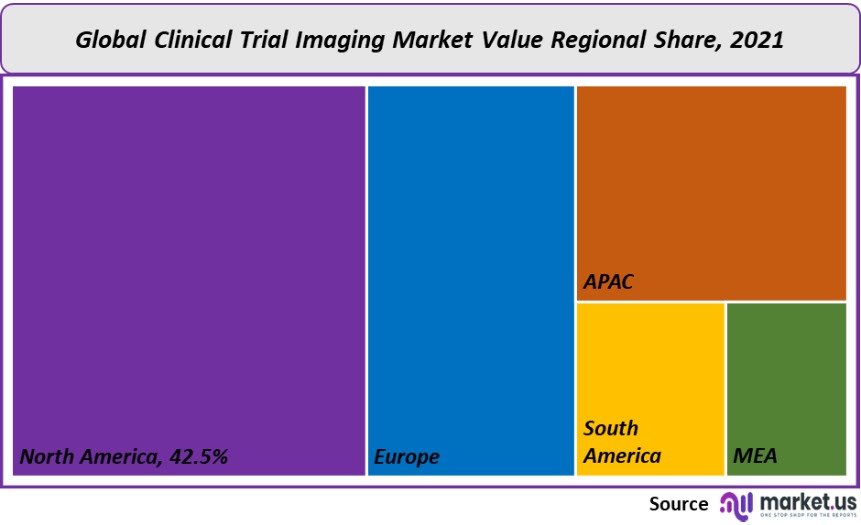
North America held a dominant position in the global market, accounting for more than 42.5% of the global clinical trials imaging market by 2021. This is due to a large number of outsourcing companies and the increase in R&D within the region. The market will be driven by a variety of factors, including the growing geriatric population and chronic diseases. North America has the highest number of clinical trials. Outsourcing activities are dominated by North America. Another factor that influences the outsourcing of clinical trials is cost.
Europe’s clinical trial imaging market is being driven by factors like the growing geriatric population, and the rising prevalence of chronic diseases, such as Huntington’s and Parkinson’s. These are driving the adoption and growth of clinical trials in Europe.
The adoption of imaging in clinical trial trials is also being driven by the fact that research laboratories are trying to cut operational costs. Each year, approximately 4,000 clinical trials for medicines are approved in the European Union.
While most of these trials are carried out in Western European countries (EU), the number of trials for medicines in the region is decreasing. The Asia Pacific will experience the fastest growth in clinical trial imaging during the forecast period. This is due to rapid growth in the region’s population, the increased R&D activity, and the growing demand for better therapies.

Key Regions and Countries covered іn thе rероrt:
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Russia
- Spain
- Rest of Europe
- APAC
- China
- Japan
- South Korea
- India
- Rest of Asia-Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- MEA
- GCC
- South Africa
- Israel
- Rest of MEA
Market Share & Key Players Analysis:
Parexel International Corporation and Intrinsic Imaging are key players in the market. These market players focus on growth strategies like mergers and acquisitions, as well as the signing of contracts. ICON plc purchased MedPass International in February 2020.
MedPass International is a European medical device CRO, reimbursement, and regulatory consulting company. The acquisition is believed to have contributed to the expansion of ICON’s European medical device and diagnostic research service.
Similar to the above, Bioclinica launched the SMART technology suite with Medical Imaging, Interactive Response Technology, (IRT), and Electronic Data Capture in June 2018. This suite allows users to submit, manage and investigate medical image data, as well as report on and transfer it, compliant with global data privacy requirements and universal data privacy.

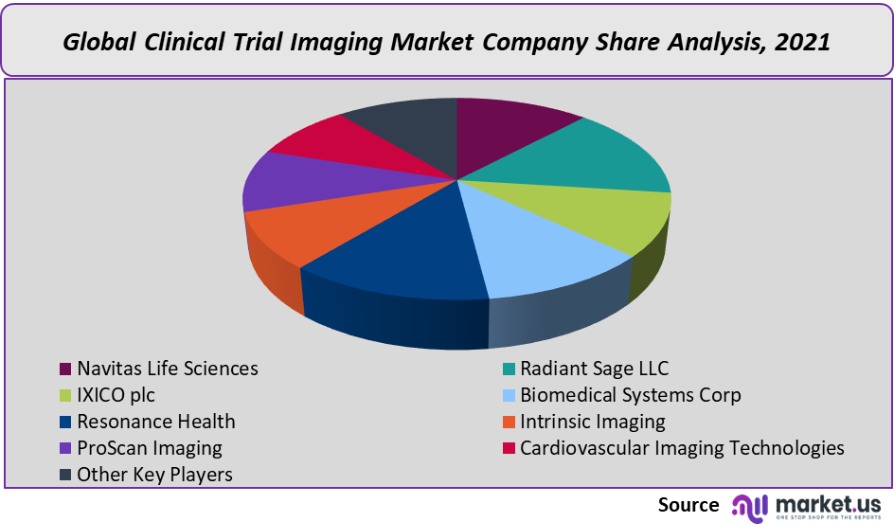
Маrkеt Кеу Рlауеrѕ:
- Navitas Life Sciences
- Radiant Sage LLC
- IXICO plc
- Biomedical Systems Corp
- Resonance Health
- Intrinsic Imaging
- ProScan Imaging
- Cardiovascular Imaging Technologies
- Other Key Players
For the Clinical Trial Imaging Market research study, the following years have been considered to estimate the market size:
Attribute Report Details Historical Years
2016-2020
Base Year
2021
Estimated Year
2022
Short Term Projection Year
2028
Projected Year
2023
Long Term Projection Year
2032
Report Coverage
Competitive Landscape, Revenue analysis, Company Share Analysis, Manufacturers Analysis, Volume by Manufacturers, Key Segments, Key company analysis, Market Trends, Distribution Channel, Market Dynamics, COVID-19 Impact Analysis, strategy for existing players to grab maximum market share, and more.
Regional Scope
North America, Europe, Asia-Pacific, South America, Middle East & Africa
Country Scope
United States, Canada and Mexico, Germany, France, UK, Russia and Italy, China, Japan, Korea, India and Southeast Asia, Brazil, Argentina, Colombia etc.Saudi Arabia, UAE, Egypt, Nigeria and South Africa
Frequently Asked Questions (FAQ)
Q: What is the size of the Clinical Trial Imaging market in 2021?A: The Clinical Trial Imaging market size is US$ 961.2 million in 2021.
Q: What is the projected CAGR at which the Clinical Trial Imaging market is expected to grow at?A: The Clinical Trial Imaging market is expected to grow at a CAGR of 7.8% (2023-2032).
Q: List the segments encompassed in this report on the Clinical Trial Imaging market?A: Market.US has segmented the Clinical Trial Imaging market by geographic (North America, Europe, APAC, South America, and Middle East and Africa). By Product and service, market has been segmented into Service and Software. By End User, the market has been further divided into Рhаrmасеutісаl Соmраnіеѕ, Віоtесhnоlоgу Соmраnіеѕ, Меdісаl Dеvісе Маnufасturеr, Соntrасt Rеѕеаrсh Оrgаnіzаtіоnѕ.
Q: List the key industry players of the Clinical Trial Imaging market?A: Navitas Life Sciences, Radiant Sage LLC, IXICO plc, Biomedical Systems Corp, Resonance Health, Intrinsic Imaging, ProScan Imaging, Cardiovascular Imaging Technologies, Other Key Players, are engaged in the Clinical Trial Imaging market.
Q: Which region is more appealing for vendors employed in the Clinical Trial Imaging market?A: North America accounted for the highest revenue share of 42.5%. Therefore, the Clinical Trial Imaging industry in North America is expected to garner significant business opportunities over the forecast period.
Q: Name the key areas of business for Pigment Dispersion?A: U.S., Canada, U.K., Germany, France, Italy, Spain & Japan, are key areas of operation for Clinical Trial Imaging Market.
Q: Which segment accounts for the greatest market share in the Clinical Trial Imaging industry?A: With respect to the Clinical Trial Imaging industry, vendors can expect to leverage greater prospective business opportunities through the service segment, as this area of interest accounts for the largest market share.
![Clinical Trial Imaging Market Clinical Trial Imaging Market]() Clinical Trial Imaging MarketPublished date: Feb 2022add_shopping_cartBuy Now get_appDownload Sample
Clinical Trial Imaging MarketPublished date: Feb 2022add_shopping_cartBuy Now get_appDownload Sample - Navitas Life Sciences
- Radiant Sage LLC
- IXICO plc
- Biomedical Systems Corp
- Resonance Health
- Intrinsic Imaging
- ProScan Imaging
- Cardiovascular Imaging Technologies
- Other Key Players
- settingsSettings
Our Clients
|
Single User
$5,999
$2,999
USD / per unit
save 50% |
Multi User
$7,999
$3,499
USD / per unit
save 55% |
Corporate User
$12,999
$4,499
USD / per unit
save 65% | |
|---|---|---|---|
| e-Access | |||
| Data Set (Excel) | |||
| Company Profile Library Access | |||
| Interactive Dashboard | |||
| Free Custumization | No | up to 10 hrs work | up to 30 hrs work |
| Accessibility | 1 User | 2-5 User | Unlimited |
| Analyst Support | up to 20 hrs | up to 40 hrs | up to 50 hrs |
| Benefit | Up to 20% off on next purchase | Up to 25% off on next purchase | Up to 30% off on next purchase |
| Buy Now ($ 2,999) | Buy Now ($ 3,499) | Buy Now ($ 4,499) |