Global Influenza Diagnostics Market By Test Type (RIDT, Cell Culture, RT-PCR, and Other Test Types), By End-Use (Hospitals, Laboratories, and Point of Care (POCT)), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2022-2032
- Published date: Nov 2021
- Report ID: 16459
- Number of Pages: 269
- Format:
- keyboard_arrow_up
Influenza Diagnostics Market Overview:
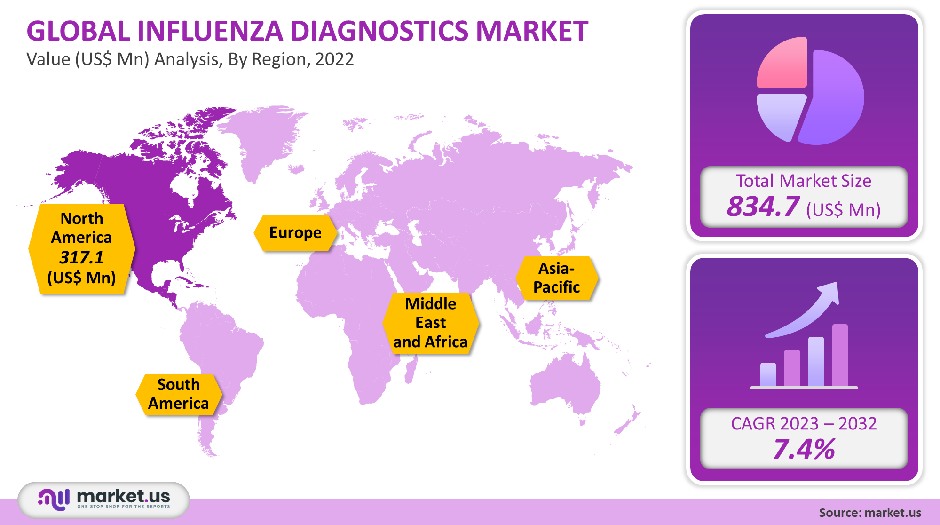
The global market for Influenza Diagnostics was worth USD 834.7 million in 2021. It is forecast to see a 7.4% CAGR during the forecast period.
Influenza, commonly known as the flu or upper respiratory infection caused by the influenza virus, is a disease that affects the upper respiratory system. Three types of seasonal influenza virus exist influenza B, influenza A, and influenza C. They are distinguished by two major surface proteins: neuraminidase (NA) and hemagglutinin (HA). Different levels of these proteins determine which serotypes can be identified.
Global Influenza Diagnostics Market Analysis
Test Type Analysis
Because they are used frequently for diagnosing influenza, rapid influenza diagnostic tests (RIDT) constituted the largest segment. These tests are used to detect influenza types A and B in specimens. Because of its rapid results, RIDT has been considered the gold standard for the diagnosis and treatment of influenza. This makes RIDT the leading test in influenza diagnostics.
Others were the holders of the second largest test type share. This is due in part to the precision and accuracy offered by serology and immunofluorescence. Immunofluorescence refers to immune-fluorescent staining respiratory specimens. Tests such as complement fixation or hem agglutination inhibition are used to diagnose the influenza virus. Automated serology utilizes enzyme-linked immunosorbent assay. These tests are preferred by medical personnel because of the lack of false positives. This has helped to accelerate their market growth.
Cell culture is often used when the disease prevalence is being determined. Cell culture allows for accurate statistics as well as the ability to distinguish between influenza types. These tests are typically performed in laboratories. The results can take several days depending on the circumstances. The diagnostic method determines whether the patient has PPV or negative predictive value. Cell culture-derived influenza vaccines have recently been produced in response to the growing need for better methods of protection against influenza.
RTPCR is expected to have the second highest CAGR of all influenza diagnostics. RTPCRs provide results in a short time. These tests are capable of detecting both inactive and viable influenza viruses. The potential power of these tests, which can determine the influenza virus type (A or B viruses), is driving the RTPCR market. Also, false positives and negative results are very rare. RTPCR has a disadvantage. It is more expensive than other influenza tests, and not all tests have been approved by FDA.

End Use Analysis
Although a high market share was held by the hospitals for influenza diagnostics, it is expected that this market will slowly decline with the introduction of POCT labs that can provide precise results. The fastest growing segment of POCTs will be due to factors like ease of use and use by anyone. They also don’t require much operational knowledge and do not require any special expertise.
These tests have seen a significant increase in trust from both physicians and the general public since the advent of highly-technical POCT. However, the market for laboratory influenza diagnostics will grow at a steady rate over the course of the study.
Hospitals can also be called long-term care institutions. As most people depend upon them to diagnose their influenza, they are the primary end-use segment. In developing and poor countries, where there is no access to rapid diagnostic testing, hospitals are the primary testing facilities.
POCT can only be done in non-laboratory environments. It is also known by the name “bedside testing”. POCT is responsible for the increase in influenza diagnostic testing kit usage. The POCT market will be the fastest growing segment during the study period.
The Real-time RTPCR technique is used for laboratory diagnosis of Influenza. Laboratories provide many benefits: They are quick, specific, specific, scalable, and cost-efficient, and can test many types of samples. Due to their limited use, the growth of laboratories is expected to be slower than that of POCT and hospitals.
Key Market Segments
By Test Type
- RIDT
- Cell Culture
- RT-PCR
- Other Test Types
By End-Use
- Hospitals
- Laboratories
- Point of Care (POCT)
Market Dynamics
Because influenza is highly contagious, the disease can be easily spread to others through infected individuals who are carriers. There are many regions that experience seasonal influenza outbreaks. Tropical regions are prone to influenza outbreaks throughout the year. This makes it difficult for tropical regions to treat the disease.
Children, elderly people, pregnant women, patients with immunosuppression, those who have chronic medical conditions, and health care workers are all at the highest risk of contracting influenza. Many antiviral drugs are used to treat influenza. They reduce the risk of complications and death. Most patients are unable to take antiviral medications because they are resistant to them. This has led to increased demand for pharmaceuticals and vaccines, which in turn has helped industry developers.
Rising geriatric populations, rising government efforts to counter influenza, and increased prevalence of chronic illnesses such as diabetes, heart diseases, and blood disorders, are just some of the factors driving the rise in the influenza diagnostics market.
Geriatrics are vulnerable to a variety of diseases because they are unable to fight off disease and are therefore at high risk for contracting influenza. People with a chronic illness or immunosuppression are more susceptible to the disease. With an increasing number of people being at risk for the disease, market growth is accelerating.
Vaccination is a key method of protecting individuals from the spread of infectious diseases. It can also prevent serious complications in patients with immunosuppression or who are at high risk. Mutations in the virus type can prove to be a problem, making it more difficult for individuals to get vaccinated. With the changes in virus types, it is essential to identify patients and give timely medication to prevent death. Therefore, market growth has been accelerated by the rising demand for influenza diagnostics.
Regional Analysis
North America accounted for the largest market share of 38% in the influenza diagnosis market. This is due to the U.S. CDC’s strong focus on controlling disease rates. High technological advancements and rising healthcare spending will drive market growth. The Asia Pacific will be the region with the fastest growth rate due to factors such as high population density, significant disease burden, and large region.
The U.S. CDC actively implements preventive measures and monitors the disease via its national Influenza Surveillance System. This helps to determine the severity of the illness and maintain a check on the disease rates. A large number of rapid diagnostic tests (RDTs), has also helped to increase adoption in the United States. North America was the region leader in market share in 2021 due to high public awareness combined with the wide availability of diagnostic procedures.
The Asia Pacific region is forecast to experience fast-paced growth in the future. Market growth is expected to be driven by increased awareness and adoption of technology. Human infection with Avian Influenza A (H5NI), which caused an influenza pandemic in Asia, is one of Asia’s most worrying factors. There is increasing concern about the spread of the disease and countries are taking active steps to prevent it.

Key Regions and Countries covered in the report:
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Russia
- Spain
- Rest of Europe
- APAC
- China
- Japan
- South Korea
- India
- Rest of Asia-Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- MEA
- GCC
- South Africa
- Israel
- Rest of MEA
Market Share & Key Players Analysis:
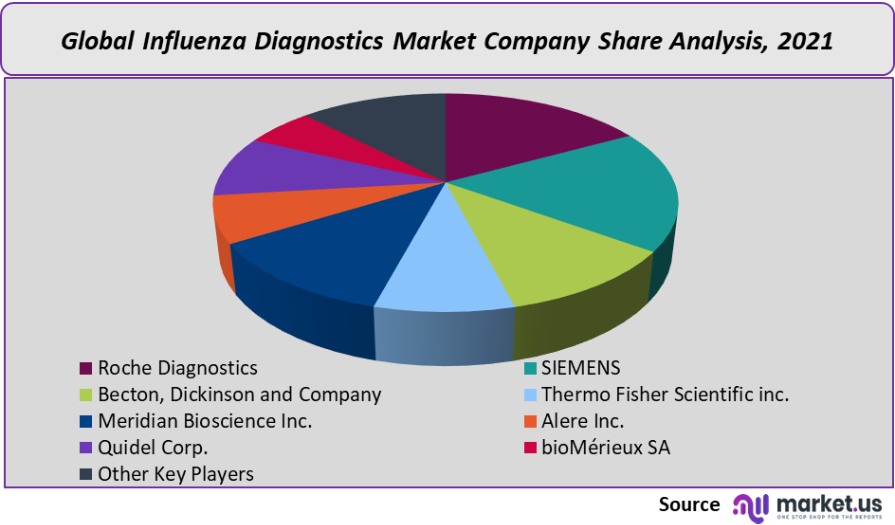
Roche Diagnostics, Becton, Thermo Fisher Scientific, Dickinson and Company, Alere, and Quidel Corporation are just a few of the players in the influenza diagnostics industry. These companies continue to work hard on developing cost-effective devices and advanced technology in order to offer effective treatment.
Alere, for instance, announced that it had signed a contract with the U.S. Department of Health and Human Services Biomedical Research. This authority is known as Biomedical Advanced Research and Design Authority (BARDA). It will develop countermeasures against the influenza virus.
Roche also received FDA clearance and a CLIA waiver to test the Cobas Influenza A/B and RSV. This test is intended to be used with cobas Liat System. Roche is the first company to broaden the scope of the test.

Маrkеt Кеу Рlауеrѕ:
- Roche Diagnostics
- SIEMENS
- Becton, Dickinson and Company
- Thermo Fisher Scientific Inc.
- Meridian Bioscience Inc.
- Alere Inc.
- Quidel Corp.
- bioMérieux SA
- Other Key Players
For the Influenza Diagnostics Market research study, the following years have been considered to estimate the market size:
Attribute Report Details Historical Years
2016-2020
Base Year
2021
Estimated Year
2022
Short Term Projection Year
2028
Projected Year
2023
Long Term Projection Year
2032
Report Coverage
Competitive Landscape, Revenue analysis, Company Share Analysis, Manufacturers Analysis, Volume by Manufacturers, Key Segments, Key company analysis, Market Trends, Distribution Channel, Market Dynamics, COVID-19 Impact Analysis, strategy for existing players to grab maximum market share, and more.
Regional Scope
North America, Europe, Asia-Pacific, South America, Middle East & Africa
Country Scope
United States, Canada and Mexico, Germany, France, UK, Russia and Italy, China, Japan, Korea, India and Southeast Asia, Brazil, Argentina, Colombia etc.Saudi Arabia, UAE, Egypt, Nigeria and South Africa
Frequently Asked Questions (FAQ)
Q: What is the size of the Influenza Diagnostics market in 2021?A: The Influenza Diagnostics market size is US$ 834.7 million in 2021.
Q: What is the projected CAGR at which the Influenza Diagnostics market is expected to grow at?A: The Influenza Diagnostics market is expected to grow at a CAGR of 7.4% (2023-2032).
Q: List the segments encompassed in this report on the Influenza Diagnostics market?A: Market.US has segmented the Influenza Diagnostics market by geographic (North America, Europe, APAC, South America, and Middle East and Africa). By Test Type, the market has been segmented into RIDT, Cell Culture, RT-PCR, and Other Test Types. By End-Use, the market has been further divided into Hospitals, Laboratories, and Point of Care (POCT).
Q: List the key industry players of the Influenza Diagnostics market?A: Roche Diagnostics, Siemens, Becton, Dickinson and Company, Thermo Fisher Scientific Inc., Meridian Bioscience Inc., Alere Inc., Quidel Corp., bioMérieux SA, Other Key Players are engaged in the Influenza Diagnostics market.
Q: Which region is more appealing for vendors employed in the Influenza Diagnostics market?A: North America is expected to account for the highest revenue share of 38%. Therefore, the Influenza Diagnostics industry in North America is expected to garner significant business opportunities over the forecast period.
Q: Name the key areas of business for Influenza Diagnostics?A: The US, Canada, UK, Japan, Mexico, India, China & Germany are key areas of operation for the Influenza Diagnostics Market.
Q: Which segment accounts for the greatest market share in the Influenza Diagnostics industry?A: With respect to the Influenza Diagnostics industry, vendors can expect to leverage greater prospective business opportunities through the RIDT segment, as this area of interest accounts for the largest market share.
![Influenza Diagnostics Market Influenza Diagnostics Market]() Influenza Diagnostics MarketPublished date: Nov 2021add_shopping_cartBuy Now get_appDownload Sample
Influenza Diagnostics MarketPublished date: Nov 2021add_shopping_cartBuy Now get_appDownload Sample - Roche Diagnostics
- Siemens Aktiengesellschaft Company Profile
- Becton, Dickinson and Company
- Thermo Fisher Scientific Company Profile
- Meridian Bioscience Inc.
- Alere Inc.
- Quidel Corp.
- bioMérieux SA
- Other Key Players
- settingsSettings
Our Clients
|
Single User
$5,999
$2,999
USD / per unit
save 50% |
Multi User
$7,999
$3,499
USD / per unit
save 55% |
Corporate User
$12,999
$4,499
USD / per unit
save 65% | |
|---|---|---|---|
| e-Access | |||
| Data Set (Excel) | |||
| Company Profile Library Access | |||
| Interactive Dashboard | |||
| Free Custumization | No | up to 10 hrs work | up to 30 hrs work |
| Accessibility | 1 User | 2-5 User | Unlimited |
| Analyst Support | up to 20 hrs | up to 40 hrs | up to 50 hrs |
| Benefit | Up to 20% off on next purchase | Up to 25% off on next purchase | Up to 30% off on next purchase |
| Buy Now ($ 2,999) | Buy Now ($ 3,499) | Buy Now ($ 4,499) |