Global Lyophilized Injectable Drugs Market by Drug Type (Herceptin IV, Keytruda, Remicade, Botox, Xolair & Other Drug Types), By Disease Indication (Autoimmune Diseases, Infectious Diseases, Metabolic Conditions & Other Disease Indications), By End-User (Hospitals, Ambulatory Surgical Centers, Specialty Clinics & Others End-Users), and by Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2022-2032
- Published date: Jun 2022
- Report ID: 42355
- Number of Pages: 242
- Format:
- keyboard_arrow_up
Lyophilized Injectable Drugs Market Overview:
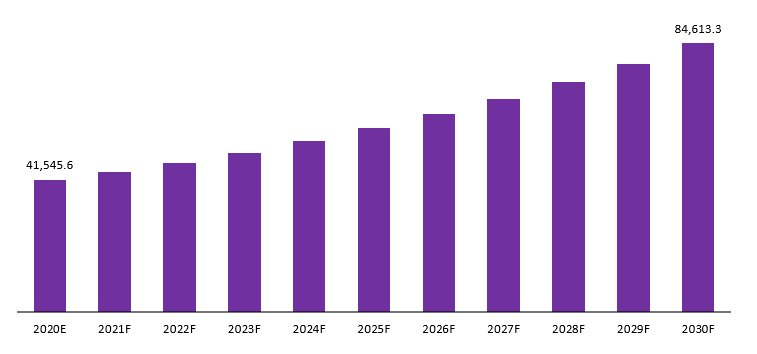
The Global Lyophilized Injectable Drugs Market is projected to be USD 41,545.6 Mn in 2020 to reach USD 84,613.3 Mn by 2029 at a CAGR of 7.5%.
Lyophilization is also known as the freeze-drying process in which the product is frozen and placed in a vacuum to remove water from the product. Lyophilization involves adjusting the solution’s temperature and pressure so that the solution phase can move directly from the frozen state to the gaseous state without moving through the liquid/state. Some typical drugs that undergo the lyophilization process include many active pharmaceutical ingredients (API), vaccines and antibodies, penicillin, blood plasma, proteins, enzymes, hormones, viruses, and bacteria.
Lyophilization can extend the shelf life, usually as long as two to five years, and reduces weight, making it easier to transport the product. The freeze-dried drug product is a lyophilized powder that can be reconstituted in a vial or prefilled syringe for self-administration by patients. Examples of freeze-dried biological products include many vaccines, such as typhoid vaccine, measles virus vaccine, cellular-derived vaccines, recombinant vaccines, and immunoglobulins. It consists of three interrelated stages, i.e., freezing, primary drying, and secondary drying.
Freezing is the first step in which water or solvent in the product gradually freezes through the cooling shelf. This creates ice crystals that separate from the drug product and are easier to remove by sublimation. In the second stage of freeze-drying, the sublimation process directly transforms solid ice into vapor without passing through the liquid phase. The vapor produced is collected by a condenser, and its temperature and pressure are lower than the product. The steam is thus converted back to ice on the surface of the condenser. The final stage of lyophilization is secondary drying (adsorption), during which ion-bound water molecules are removed by increasing the temperature above the temperature of the initial drying stage.
Global Lyophilized Injectable Drugs Market Revenue (US$ Mn), 2020–2030:

In developing countries, autoimmune diseases and infectious diseases caused by bacteria and fungi is very high. It is essential to develop an economic freeze-drying cycle suitable for the large-scale production of drugs to treat these diseases found in large quantities. Also, the increasing world population and the growing geriatric population are increasing. These factors are expected to support market growth.
The covid-19 pandemic has increased the demand for injectable drugs to administer antibiotic drugs to fight infections such as coronavirus and SARS-CoV-2 and other chronic diseases. Also, an increase in the number of siRNA and antibody-based preparations needs to be freeze-dried to maintain the integrity of siRNA targeting the body. These factors are expected to contribute to market growth and allow new players to enter the market in the forecasted period.
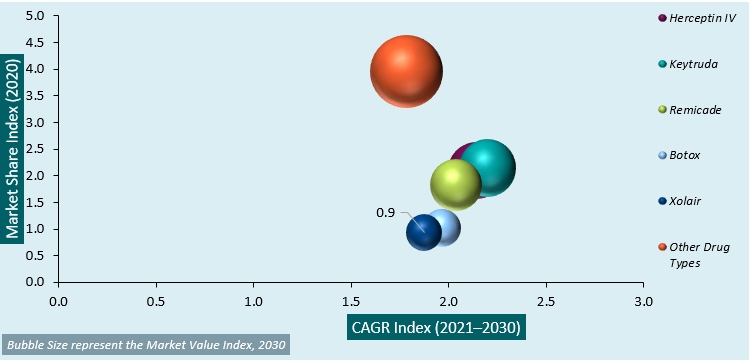
Global Lyophilized Injectable Drugs Market Attractiveness Analysis by Butter Type, 2014–2030:

The global lyophilized injectable drugs market is segmented into drug type, disease indications, end-user, and regions & countries. The drug type segments include Herceptin IV, keytruda, Remicade, botox, carimune NF, and other drug types. Among the drug type segments, the keytruda segment accounts for the highest revenue share, owing to its high adoption in treating melanoma, lung cancer, Hodgkin lymphoma, head and neck cancer, and stomach cancer. Among the disease indication segments, autoimmune diseases account for a significantly high revenue share compared to the other diseases in this category.
This is attributed to the increase in the number of immunodeficiency diseases, which reduces the body’s ability to resist invaders, resulting in infection susceptibility. Among the end-user segments, the hospital segment accounts for a significant share in terms of revenue. Among the regional markets, the market in North America is expected to account for a significant revenue share and is projected to maintain its dominance in the global lyophilized injectable drugs market over the forecast period. This is due to the high presence of manufacturers coupled with large production capabilities in the region.
The research report on the Global Lyophilized Injectable Drugs Market includes profiles of some of the major companies such as:
- Johnson & Johnson,
- Roche Holding AG,
- Novartis AG, Merck & Co., Inc.,
- Bristol Myers Squibb Co,
- Takeda Pharmaceutical Company Limited,
- Allergan Plc,
- Biogen, Inc.,
- Mylan NV,
- CIRON Group
Global Lyophilized Injectable Drugs Market is segmented based on Drug Type, Disease Indications, End-User, and Region:
Based on Drug Type
- Herceptin IV
- Keytruda
- Remicade
- Botox
- Carimune NF
- Other Drug Types
Based on Disease Indication
- Autoimmune Diseases
- Infectious Diseases
- Metabolic Conditions
- Other Disease Indications
Based on End-User
- Hospitals
- Ambulatory Surgical Centers
- Specialty Clinics
- Other End-Users
Based on Region
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
For the Lyophilized Injectable Drugs Market research study, the following years have been considered to estimate the market size:
Attribute Report Details Historical Years
2016-2020
Base Year
2021
Estimated Year
2022
Short Term Projection Year
2028
Projected Year
2023
Long Term Projection Year
2032
Report Coverage
Competitive Landscape, Revenue analysis, Company Share Analysis, Manufacturers Analysis, Volume by Manufacturers, Key Segments, Key company analysis, Market Trends, Distribution Channel, Market Dynamics, COVID-19 Impact Analysis, strategy for existing players to grab maximum market share, and more.
Regional Scope
North America, Europe, Asia-Pacific, South America, Middle East & Africa
Country Scope
United States, Canada and Mexico, Germany, France, UK, Russia and Italy, China, Japan, Korea, India and Southeast Asia, Brazil, Argentina, Colombia etc.Saudi Arabia, UAE, Egypt, Nigeria and South Africa
![Lyophilized Injectable Drugs Market Lyophilized Injectable Drugs Market]() Lyophilized Injectable Drugs MarketPublished date: Jun 2022add_shopping_cartBuy Now get_appDownload Sample
Lyophilized Injectable Drugs MarketPublished date: Jun 2022add_shopping_cartBuy Now get_appDownload Sample - Johnson & Johnson,
- Roche Holding AG,
- Novartis AG, Merck & Co., Inc.,
- Bristol Myers Squibb Co,
- Takeda Pharmaceutical Company Limited,
- Allergan Plc,
- Biogen, Inc.,
- Mylan NV,
- CIRON Group
- settingsSettings
Our Clients
|
Single User
$5,999
$2,999
USD / per unit
save 50% |
Multi User
$7,999
$3,499
USD / per unit
save 55% |
Corporate User
$12,999
$4,499
USD / per unit
save 65% | |
|---|---|---|---|
| e-Access | |||
| Data Set (Excel) | |||
| Company Profile Library Access | |||
| Interactive Dashboard | |||
| Free Custumization | No | up to 10 hrs work | up to 30 hrs work |
| Accessibility | 1 User | 2-5 User | Unlimited |
| Analyst Support | up to 20 hrs | up to 40 hrs | up to 50 hrs |
| Benefit | Up to 20% off on next purchase | Up to 25% off on next purchase | Up to 30% off on next purchase |
| Buy Now ($ 2,999) | Buy Now ($ 3,499) | Buy Now ($ 4,499) |