Global Materiovigilance Market, by Delivery Mode (On-premise, On-cloud) by Application (Diagnostic Application, Therapeutic Application, Surgical Application, Research Application, Other Applications), By End-User (Contract Research Organization, Business Process Outsourcing, Original Equipment Manufacturers, Other End-Users), and by Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2022-2032
- Published date: Feb 2022
- Report ID: 84594
- Number of Pages: 209
- Format:
- keyboard_arrow_up
Materiovigilance Market Overview
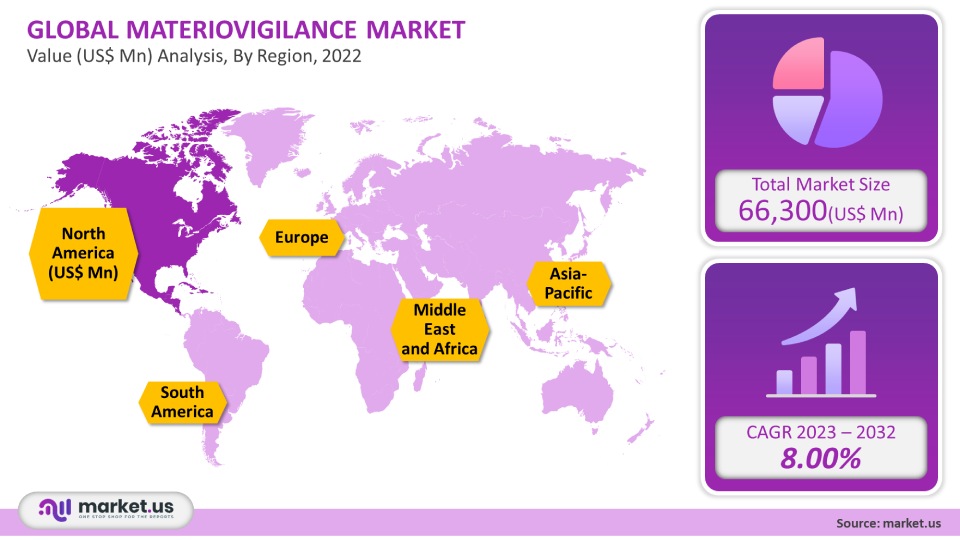
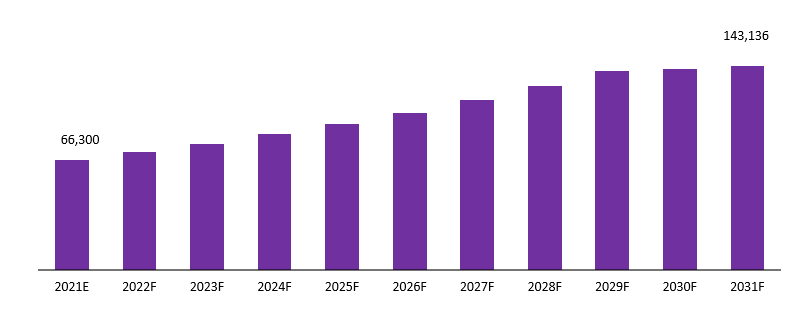
The global Materiovigilance Market is projected to reach a valuation of USD 143,136 Mn by 2031 at a CAGR of 8.00%, from USD 66,300 Mn in 2021.
“Global Materiovigilance Market, by Delivery Mode (On-premise, On-cloud) by Application (Diagnostic Application, Therapeutic Application, Surgical Application, Research Application, Other Applications), By End-User (Contract Research Organization, Business Process Outsourcing, Original Equipment Manufacturers, Other End-Users), and by Region – Global Forecast to 2031”. This report offers a holistic view of the global Materiovigilance market through systematic segmentation that covers every aspect of this target market.

Materiovigilance is the systematic and ongoing collection, analysis, and interpretation of information about the adverse effects associated with the use of the material in healthcare. Materiovigilance aims to identify, assess, and prevent any harm to patients that may be associated with medical devices and other materials. Materiovigilance activities include collecting and analyzing reports of adverse reactions and incidents and studying the use of materials in-patient care. Materiovigilance is the practice of systematically monitoring the safety of medical devices and implants throughout their lifecycle. Materiovigilance aims to ensure that medical devices and implants are safe for patients and do not cause any harm. Materiovigilance is a relatively new field, and there is still much to learn about it.
While Materiovigilance is an important tool to protect patients, it has several limitations. One limitation is the lack of a coordinated system for reporting adverse events. This can make it difficult to track and analyze potential safety issues.
Various medical gadgets are employed in the ongoing COVID-19 pandemic to prevent or treat disease. Masks, respirators, ventilators, Personal Protective Equipment (PPE) kits, in-vitro diagnostic (IVD) kits, sanitizers, and a variety of other items are among them. According to various media reports, counterfeit and low-quality medical gadgets are widely available in the market, posing a severe health danger to patients and healthcare providers. As a result, stringent vigilance of medical device surveillance is essential to prevent the use of medical devices that do not satisfy the minimum quality requirements. Manufacturers or authorized agencies can recall batches of such medical products from the market. A recall is any action taken by a medical device’s manufacturer or supplier to remove or withdraw a medical device from the market or collect a medical device from any person it has been delivered due to health risks.
Global Materiovigilance Market Revenue (US$ Mn), 2021–2031

Source: Prudour, 2021
Over the projected timeline, the global Materiovigilance market is expected to develop due to an increase in medical device recalls due to technical faults. According to data published by Medical Device and Diagnostic Industry (MD+DI), which is a resource exclusively for original equipment manufacturers of medical devices and in-vitro diagnostic products, the number of medical device recalls issued in the first half of 2021 was higher than the first half of each of the previous five years in June 2021. The US Food and Drug Administration (US FDA) recorded 29 medical device recalls in the first half of 2021. This factor is anticipated to aid in augmenting this market in future years.
Well-known companies in the Materiovigilance industry lack skilled workers, which impacts their work culture’s efficiency. As a result of these considerations, the Materiovigilance market is expected to face certain challenges in its growth prospects in the upcoming years. According to data published in 2016 by the Organization for Economic Cooperation and Development (OECD), nearly 50% of Latin American formal enterprises cannot find staff with the skills they want, compared to 36% of firms in OECD countries. Additionally, the safety rules of goods are comparably complicated. Furthermore, in emerging or developing economies, a scarcity of experienced specialists is a significant barrier limiting the growth potential of the Materiovigilance market.
The competitors in the Materiovigilance market are focusing their efforts on bringing technological breakthroughs through increased R&D activities. These businesses are adopting various market strategies to establish expansion opportunities in the coming years. These growth strategies are just a few of portfolio diversification, the expansion of multiple distribution networks, product creation and launch, mergers & acquisitions, and expanding an organization’s footprints with subsidiaries and partnerships.
Veeva Systems Inc., for example, has stated that Veeva Vault Product Surveillance would be available in April 2020. The firm is a market leader in cloud-based software. The application is now available for use in diagnostics and medical devices. Factors such as this are expected to create lucrative opportunities for existing and emerging players in this market.
The market is indexing an increase in hospital penetration around the world to address the changing medical needs of patients and the availability of new medical equipment. Governments worldwide are also investing extensively in the construction of hospitals, both in developed and emerging countries, to satisfy the growing requirements of patients and provide safe and reliable health solutions. For example, the India Brand Equity Foundation (IBEF) revealed that the Indian government announced a US$9.87 billion investment in the healthcare industry in its Union Budget 2020-21. By 2022, the government wants to raise healthcare spending to 3% of the overall GDP.
This research report on the global Materiovigilance market includes major company profiles such as
- AssurX
- Sparta Systems
- Oracle Corporation
- Xybion Corporation
- Sarjen Systems Pvt. Ltd.
- MDI Consultants
- AB-Cube
- Q Vigilance
- Qserve
- ZEINCRO, among others.
Global Materiovigilance Market Segmentation Based on Delivery Mode, Application, End-User, and Region
Based on Delivery Mode
- On-Premise
- On-Cloud
Based on Application
- Diagnostic Application
- Therapeutic Application
- Surgical Application
- Research Application
- Other Applications
Based on End-User
- Contract Research Organization
- Business Process Outsourcing
- Original Equipment Manufacturers
- Other End-Users
Based on Region
- North America
- Europe
- Asia-Pacifica
- South America
- Middle East & Africa
For the Materiovigilance Маrkеt research study, the following years have been considered to estimate the market size:
Attribute Report Details Historical Years
2016-2020
Base Year
2021
Estimated Year
2022
Short Term Projection Year
2028
Projected Year
2023
Long Term Projection Year
2032
Report Coverage
Competitive Landscape, Revenue analysis, Company Share Analysis, Manufacturers Analysis, Volume by Manufacturers, Key Segments, Key company analysis, Market Trends, Distribution Channel, Market Dynamics, COVID-19 Impact Analysis, strategy for existing players to grab maximum market share, and more.
Regional Scope
North America, Europe, Asia-Pacific, South America, Middle East & Africa
Country Scope
United States, Canada and Mexico, Germany, France, UK, Russia and Italy, China, Japan, Korea, India and Southeast Asia, Brazil, Argentina, Colombia etc.Saudi Arabia, UAE, Egypt, Nigeria and South Africa
![Materiovigilance Market Materiovigilance Market]()
- settingsSettings
Our Clients
|
Single User
$5,999
$2,999
USD / per unit
save 50% |
Multi User
$7,999
$3,499
USD / per unit
save 55% |
Corporate User
$12,999
$4,499
USD / per unit
save 65% | |
|---|---|---|---|
| e-Access | |||
| Data Set (Excel) | |||
| Company Profile Library Access | |||
| Interactive Dashboard | |||
| Free Custumization | No | up to 10 hrs work | up to 30 hrs work |
| Accessibility | 1 User | 2-5 User | Unlimited |
| Analyst Support | up to 20 hrs | up to 40 hrs | up to 50 hrs |
| Benefit | Up to 20% off on next purchase | Up to 25% off on next purchase | Up to 30% off on next purchase |
| Buy Now ($ 2,999) | Buy Now ($ 3,499) | Buy Now ($ 4,499) |