Global Medical Devices Vigilance Market, by Delivery Mode (On-Demand/Cloud-Based (SAAS) Delivery Mode, On-Premises Delivery Mode) by Application (Diagnostic Center, Therapeutic Center, Surgical Center, Research Center, Other Applications), and by Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2022-2032
- Published date: Feb 2022
- Report ID: 84635
- Number of Pages: 318
- Format:
- keyboard_arrow_up
Medical Devises Vigilance Market Overview
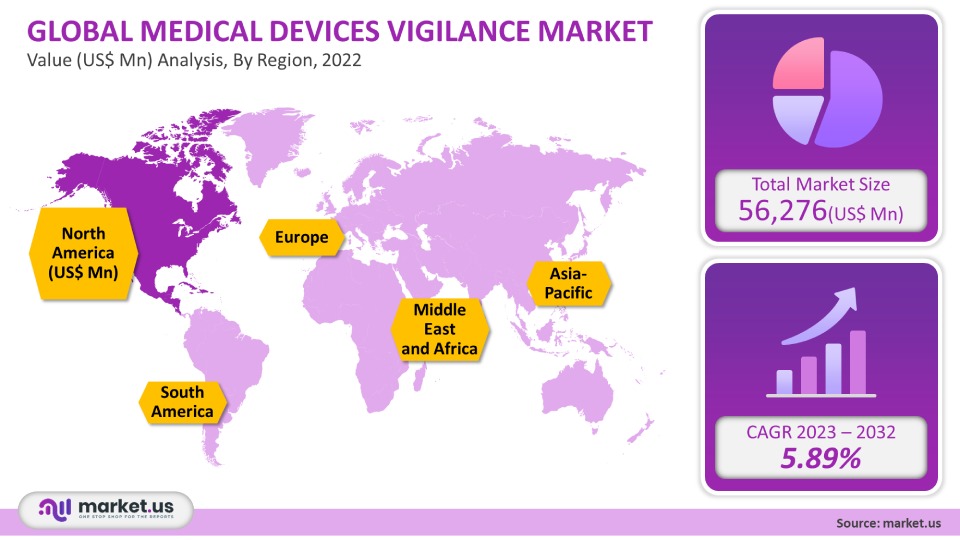
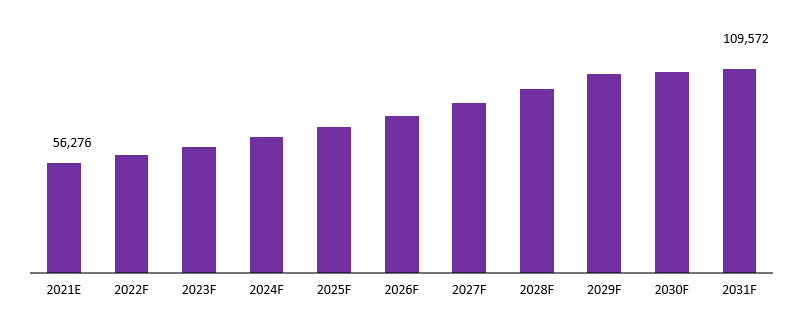
The global medical devices vigilance market is projected to reach a valuation of USD 109,572 Mn by 2031 at a CAGR of 5.89%, from USD 56,276 Mn in 2021.
“Global Medical Devices Vigilance Market, by Delivery Mode (On-Demand/Cloud-Based (SAAS) Delivery Mode, On-Premises Delivery Mode) by Application (Diagnostic Center, Therapeutic Center, Surgical Center, Research Center, Other Applications), By End-User (Medical Devices Manufacturers, Clinical Research Organizations (CROs), Business Processes Outsourcing (BPOs)), and by Region – Global Forecast to 2031”. This report offers a holistic view of the global medical devices vigilance market through systematic segmentation that covers every aspect of this target market.

Medical devices span a wide range of products, from diagnostic medical devices used to detect diseases and anomalies to therapeutic medical devices used to cut tissue, cover wounds, or close open clogged arteries, to extremely complicated and computerized medical equipment. Because of their diversity and the crucial necessity for therapeutic treatment and patient care, it is becoming increasingly important to regulate the manufacturing, operating, and distribution processes to assure their quality, safety, and efficiency. The collection, evaluation, reporting, quality check, and recognition of the condition of medical devices as a result of their use is known as medical device vigilance or materiovigilance. Medical device vigilance attempts to protect patients, healthcare facilities that use medical devices, and others by preventing or reducing the occurrence of medical device-related incidents. Medical device vigilance monitors the detection of technical faults and potential adverse effects linked to medical equipment.
Medical devices such as prosthetic limbs and artificial organs are often state-of-the-art and can be very expensive. Consequently, these devices’ safety is vital to patient’s safety. The FDA monitors any device considered “high risk,” such as medical devices implanted in the human body and drug delivery systems, and requires manufacturers to register these products with the FDA. Medical devices are very important to patient care, with over 20% of hospital expenditures being spent on them. Patients may use these devices for weeks or years, but they are often left untested for safety and efficacy before coming into the market or being adopted by hospitals. The FDA does not have enough resources to keep up with the ever-growing number of new devices, leaving their safety to patients’ trust in manufacturers.
The Office of Medical Devices Vigilance (OMDV) is a branch of the FDA that conducts investigations to ensure the safety and efficacy of medical devices. The FDA relies on patient reports, hospital reports, injury or event reports, and submitted samples when investigating medical devices. A submitted sample could be anything from blood to skin samples. OMDV also investigates audits made by manufacturers when questions arise about the safety or efficacy of their products.
Global Medical Devices Vigilance Market Revenue (US$ Mn), 2021–2031

Source: Prudour, 2021
The primary factor complementing the global market expansion for medical device vigilance is an increase in the number of medical devices due to an increase in the number of hospitals and an increase in the number of medical devices recalled due to flaws and safety issues. According to the FDA, about 1.4 million adverse occurrences were reported to the agency in the United States and transferred to the FDA via the medical device reporting system. In addition, a surge in the adoption of medical device surveillance systems, growing awareness of regulatory safety guidelines, and the availability of medical device vigilance software to ensure compliance with these regulations, as well as an increase in adverse event reporting, are expected to significantly accelerate the medical device vigilance industry’s growth in the coming years. The medical device vigilance market is being propelled by several factors, including an increase in the number of medical devices recalled due to technical defects, an increase in the number of post-market surveillance programs, an increase in the incidences of adverse events related to faulty medical devices, an increase in the use of medical devices with an increase in the number of hospitals, and the adoption of medical device vigilance software. In April 2017, the Ministry of Food and Drug Safety (MFDS) of South Korea launched the ‘Harmful Medical Device Distribution Shutdown Mechanism,’ a new medical device recall system. Furthermore, increasing public awareness concerning the availability of medical device vigilance software and the reporting of adverse events, as well as stringent mandates of safety regulation post-commercialization of medical devices by various regulatory authorities around the world, such as the United States Food and Drug Administration (FDA), the Australian Therapeutic Goods Administration (TGA), and the European Medicines Agency (EMA). As a result, the market for medical device vigilance is anticipated to expand in tandem with the increasing complexity and implementation of stringent patient safety requirements. These are certain factors that are expected to aid in augmenting this market in the years to come.
On the other hand, manufacturing businesses’ disregard for product safety, a scarcity of experienced specialists in underdeveloped and developing countries, and the increasing complexity of safety regulations are slated to stymie this market’s expansion. Moreover, funding for R&D activities linked to medical device vigilance has been reduced due to the medical field’s focus on treating COVID-19. This segment has been subjected to carelessness, negatively impacting the medical device vigilance market.
This research report on the global medical devices vigilance market includes major company profiles such as
- ZEINCRO
- AssurX Inc.
- Sparta Systems
- Oracle Corporation
- Xybion Corporation
- Sarjen Systems Pvt. Ltd.
- MDI Consultants Inc.
- AB-Cube, Laerdal Medical
- Omnify Software Inc., among others.
Global Medical Devices Vigilance Market Segmentation is Based on Delivery Mode, Application, End-User, and Region
Based on Dosage Form
- On-Demand/Cloud-Based (SAAS) Delivery Mode
- On-Premises Delivery Mode
Based on Application
- Diagnostic Center
- Therapeutic Center
- Surgical Center
- Research Center
- Other Applications
Based on End-User
- Medical Devices Manufacturers
- Clinical Research Organizations (CROs)
- Business Processes Outsourcing (BPOs)
Based on Region
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
For the Medical Devices Vigilance Market research study, the following years have been considered to estimate the market size:
Attribute Report Details Historical Years
2016-2020
Base Year
2021
Estimated Year
2022
Short Term Projection Year
2028
Projected Year
2023
Long Term Projection Year
2032
Report Coverage
Competitive Landscape, Revenue analysis, Company Share Analysis, Manufacturers Analysis, Volume by Manufacturers, Key Segments, Key company analysis, Market Trends, Distribution Channel, Market Dynamics, COVID-19 Impact Analysis, strategy for existing players to grab maximum market share, and more.
Regional Scope
North America, Europe, Asia-Pacific, South America, Middle East & Africa
Country Scope
United States, Canada and Mexico, Germany, France, UK, Russia and Italy, China, Japan, Korea, India and Southeast Asia, Brazil, Argentina, Colombia etc.Saudi Arabia, UAE, Egypt, Nigeria and South Africa
![Medical Devices Vigilance Market Medical Devices Vigilance Market]() Medical Devices Vigilance MarketPublished date: Feb 2022add_shopping_cartBuy Now get_appDownload Sample
Medical Devices Vigilance MarketPublished date: Feb 2022add_shopping_cartBuy Now get_appDownload Sample - ZEINCRO
- AssurX Inc.
- Sparta Systems
- Oracle Corporation
- Xybion Corporation
- Sarjen Systems Pvt. Ltd.
- MDI Consultants Inc.
- AB-Cube, Laerdal Medical
- Omnify Software Inc., among others
- settingsSettings
Our Clients
|
Single User
$5,999
$2,999
USD / per unit
save 50% |
Multi User
$7,999
$3,499
USD / per unit
save 55% |
Corporate User
$12,999
$4,499
USD / per unit
save 65% | |
|---|---|---|---|
| e-Access | |||
| Data Set (Excel) | |||
| Company Profile Library Access | |||
| Interactive Dashboard | |||
| Free Custumization | No | up to 10 hrs work | up to 30 hrs work |
| Accessibility | 1 User | 2-5 User | Unlimited |
| Analyst Support | up to 20 hrs | up to 40 hrs | up to 50 hrs |
| Benefit | Up to 20% off on next purchase | Up to 25% off on next purchase | Up to 30% off on next purchase |
| Buy Now ($ 2,999) | Buy Now ($ 3,499) | Buy Now ($ 4,499) |