Global Mri Compatible Iv Infusion Pumps Market By Type (Nоn-Mаgnеtіс Рumрѕ, Magnetic Рumр Ѕуѕtеm With Ѕhіеldіng) By End-User (Hospitals, Ambulatory Surgical Centres, Diagnostics And Imaging Centres), By Region, And Key Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends And Forecast 2021-2031
- Published date: Mar 2022
- Report ID: 28040
- Number of Pages: 222
- Format:
- keyboard_arrow_up
Global MRI Compatible IV Infusion Pumps Market is expected to be driven
Market.US announces publication of its most recently generated research report titled, “Global MRI Compatible IV Infusion Pumps Market by Type (Nоn-mаgnеtіс Рumрѕ, and Magnetic Рumр Ѕуѕtеm with Ѕhіеldіng) by End-User (Hospitals, Ambulatory Surgical Centres, and Diagnostics and Imaging Centres) – Global Forecast to 2031”, which offers a holistic view of Global MRI Compatible IV Infusion Pumps Market through systematic segmentation that covers every aspect of the target market.
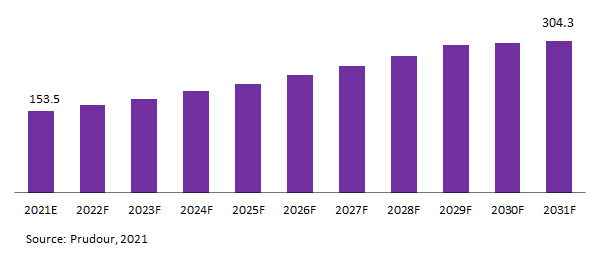
Global MRI Compatible IV Infusion Pumps Market is projected to be US$ 153.5 Mn in 2021 to reach US$ 304.3.7 Mn by 2031 at a CAGR of 7.2%.
Magnetic Resonance Imaging (MRI) is a widely-used medical imaging technology to visualize vital organs, bodily functions, as well as to identify blockages, abnormalities, and unwanted tissue growth. Generally considered safer than other scanning techniques, MRIs are finding new applications in cardiac stress testing, intra-operative MRI surgeries, and neurology. However, the powerful magnets used in MRI scanners can present certain challenges for medical facilities and imaging centers.
Most medical devices are made with magnetic components and may not function properly in the presence of a MRI scanner. There’s also a risk that items containing ferrous metals may become projectiles due to the strong magnetic field produced by The MRI scanner, posing risks for both the patient and staff. The presence of electronic instruments nearby may also negatively affect an MRI’s imaging quality. Hospitals sometimes avoid these risks by removing patients from infusion pumps and vital signs monitors during MRI procedures.
trending_up Total Revenue in 2018$ 153.5 Mn
trending_up Market CAGR of the Next Ten Years7.2%
no_encryption Market Value (US$ Mn), Share (%) and Growth Rate (%) Comparison 2012-2028Purchase this report or a membership to unlock the market value (US$ Mn), share (%) and growth rate (%) comparison for this industry.- By Type
- By Region
- By Application
no_encryption Leading Companies Financial HighlightsPurchase this report or a membership to unlock the leading companies financial highlights for this industry.trending_up Market Revenue of the Next Ten Years$ 304.3.7 Mn
Critically ill patients should not be removed from IV medications even for a few minutes, if removed their physical condition may deteriorate due to pain, cardiac distress, breathing difficulties, or other adverse outcomes that may go undetected by a vital signs monitor. In addition, several patients, particularly children and infants require continuous sedation to remain still during an MRI scan. The days of transferring a patient from traditional transport equipment to The MRI monitor & MRI IV pump is now a thing of the past.
Non-magnetic infusion pumps are small, lightweight, easy-to-use, and are designed to travel with a patient between an MRI suite and their respective care unit. These unique attributes increase an MRIs efficiency while decreasing the amount of time critically ill patients are away from their given care units.
Transferring a patient to an MRI system from an originating department such as an ICU, an emergency department, or even an anesthesia induction room, reduces the need for unnecessary equipment transfers, by providing benefits such as reduction in the amount of time that critical patients are off a given unit, continuity of care during inter-departmental transports, the efficient use of an MRI scanner, as well as increased patient processing through an expedited MRI diagnosis.
Global MRI Compatible IV Infusion Pumps Market Revenue (US$ Mn), 2021–2031

Healthcare providers across the globe are increasingly aware of the various occupational risks that could potentially arise due to the use of magnetic MRI-compatible IV infusion pump systems. Magnetic MRI-compatible IV infusion pump systems may create safety concerns and could be harmful to patients as well as MRI operators. The magnetic components of these infusion pumps may also be responsible for variations in diagnostics and imaging.
To avoid these inherent risks and comply with related safety concerns, MRI operators prefer non-magnetic MRI-compatible IV infusion pump systems. This factor is anticipated to boost market demand for these devices, and will consequently augment revenue growth prospects for this market.
An MRI examination can influence the programming or functionality of an infusion pump, even if specific requirements have been met. In 2018, the US Food and Drug Administration received evidence of significant adverse effects including harm to a patient, as well as deaths associated with the use of implantable infusion pumps in The MRI field. Therefore, the various safety concerns regarding implantable infusion pumps are expected to limit the economic growth of The MRI infusion pumps market.
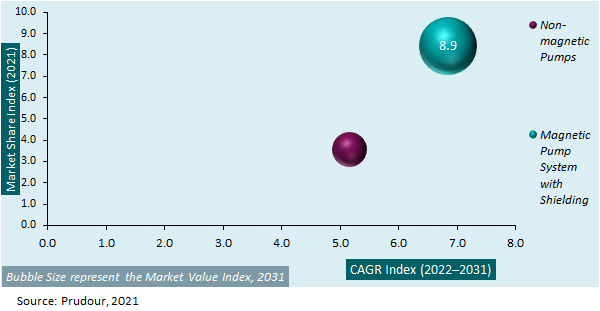
Global MRI Compatible IV Infusion Pumps Market Attractiveness Analysis by Pump Type, 2015–2031

The global MRI compatible IV infusion pumps market is segmented on the basis of pump type, end-user, and regions. In terms of pump type market segmentation, the global MRI compatible IV infusion pumps market is sub-segmented into – nоn-mаgnеtіс рumрѕ and magnetic рumр ѕуѕtеm wіth ѕhіеldіng. With respect to pump type market segmentation, the magnetic рumр ѕуѕtеm wіth ѕhіеldіng sub-segment is expected to account for a majority revenue share (70.2%) of this target market in 2021.
Based on the end-user market segmentation aspect, the global MRI compatible IV infusion pumps market is sub-segmented into – hospitals, ambulatory surgical centres and diagnostics and imaging centres. With respect to the application segmentation of this target market, the hospital sub-segment is projected to register a high rate of revenue growth over the forecast period.
On the basis of region, the global MRI compatible IV infusion pumps are segmented into North America, Europe, Asia-Pacific, South America, and the Middle East & Africa. Among these aforementioned regions, the markets in North America are expected to account for the majority revenue share of the global MRI compatible IV infusion pumps market over the forecast period.
The research report on Global MRI Compatible IV Infusion Pumps Market includes profiles of some of the major companies such as Fresenius SE & Co. KGaA (Fresenius Kalbi AG), Becton Dickinson and Company (Caesarea Medical Electronics), B. Braun Melsungen Aktiengesellschaft, Nipro Corporation, Smiths Group Plc (Smiths Medical, Inc.), KellyMed Co., Ltd., Iradimed Corporation, Medcaptain Medical Technology Co., Ltd., Flowonix Medical, Inc., Arcomed AG, MRI Devices Ltd., and other key players.
Global MRI Compatible IV Infusion Pumps Market Segmentation Based on Pump Type, End-User, and Region
Based on Pump Type:
- Nоn-mаgnеtіс Рumрѕ
- Magnetic Рumр Ѕуѕtеm wіth Ѕhіеldіng
Based on End-User:
- Hospitals
- Ambulatory Surgical Centres
- Diagnostics and Imaging Centres
Based on Key Players:
- Fresenius SE & Co. KGaA (Fresenius Kalbi AG)
- Becton Dickinson and Company (Caesarea Medical Electronics)
- B. Braun Melsungen Aktiengesellschaft
- Nipro Corporation
- Smiths Group Plc (Smiths Medical Inc.)
- KellyMed Co. Ltd.
- Iradimed Corporation
- Medcaptain Medical Technology Co. Ltd.
- Flowonix Medical Inc.
- Arcomed AG
- MRI Devices Ltd.
- other key players
Based on Regions:
- North America
- Europe
- Asia Pacific
- South America
- Middle East & Africa
For the Global MRI Compatible IV Infusion Pumps Market research study, the following years have been considered to estimate the market size:
Attribute Report Details Historical Years
2016-2020
Base Year
2021
Estimated Year
2022
Short Term Projection Year
2028
Projected Year
2023
Long Term Projection Year
2032
Report Coverage
Competitive Landscape, Revenue analysis, Company Share Analysis, Manufacturers Analysis, Volume by Manufacturers, Key Segments, Key company analysis, Market Trends, Distribution Channel, Market Dynamics, COVID-19 Impact Analysis, strategy for existing players to grab maximum market share, and more.
Regional Scope
North America, Europe, Asia-Pacific, South America, Middle East & Africa
Country Scope
United States, Canada and Mexico, Germany, France, UK, Russia and Italy, China, Japan, Korea, India and Southeast Asia, Brazil, Argentina, Colombia etc.Saudi Arabia, UAE, Egypt, Nigeria and South Africa
![MRI Compatible IV Infusion Pumps Market MRI Compatible IV Infusion Pumps Market]() MRI Compatible IV Infusion Pumps MarketPublished date: Mar 2022add_shopping_cartBuy Now get_appDownload Sample
MRI Compatible IV Infusion Pumps MarketPublished date: Mar 2022add_shopping_cartBuy Now get_appDownload Sample - Fresenius SE & Co. KGaA (Fresenius Kalbi AG)
- Becton Dickinson and Company (Caesarea Medical Electronics)
- B. Braun Melsungen Aktiengesellschaft
- Nipro Corporation Company Profile
- Smiths Group Plc (Smiths Medical Inc.)
- KellyMed Co. Ltd.
- Iradimed Corporation
- Medcaptain Medical Technology Co. Ltd.
- Flowonix Medical Inc.
- Arcomed AG
- MRI Devices Ltd.
- other key players
- settingsSettings
Our Clients
|
Single User
$5,999
$2,999
USD / per unit
save 50% |
Multi User
$7,999
$3,499
USD / per unit
save 55% |
Corporate User
$12,999
$4,499
USD / per unit
save 65% | |
|---|---|---|---|
| e-Access | |||
| Data Set (Excel) | |||
| Company Profile Library Access | |||
| Interactive Dashboard | |||
| Free Custumization | No | up to 10 hrs work | up to 30 hrs work |
| Accessibility | 1 User | 2-5 User | Unlimited |
| Analyst Support | up to 20 hrs | up to 40 hrs | up to 50 hrs |
| Benefit | Up to 20% off on next purchase | Up to 25% off on next purchase | Up to 30% off on next purchase |
| Buy Now ($ 2,999) | Buy Now ($ 3,499) | Buy Now ($ 4,499) |