Global Orphan Drugs Market, by Disease Types (Oncologic Diseases, Metabolic Diseases, Hematologic and Immunologic Diseases, Other Rare Diseases), By Disease Type (Non-Hodgkin Lymphoma, Acute Myeloid Leukemia, Other Indications), By Distribution Channels (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy) and by Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2022-2032
- Published date: Jan 2022
- Report ID: 84896
- Number of Pages: 277
- Format:
- keyboard_arrow_up
Orphan Drugs Market Overview:
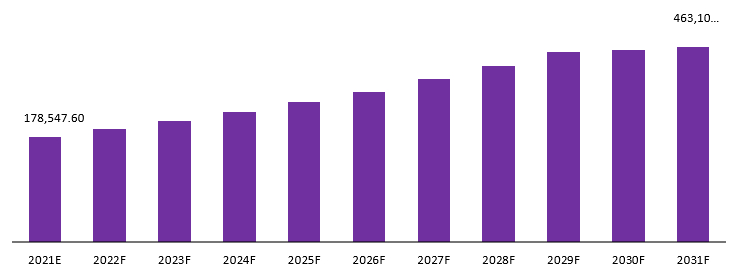
The global orphan drugs market is projected to reach a valuation of USD 535,474.85 Mn by 2032 at a CAGR of 10.5%, from USD 197,295.10 Mn in 2022.
Orphan drugs are forms of medication that target diseases and illnesses which don’t affect many people or are rare in nature. The Orphan Drug Act of 1983 facilitates the development of drugs for rare diseases. It also protects drug companies from losing money by providing them with exclusive rights to market their products for 7 years. Recently, the term orphan drug has been used to refer to medications used in the treatment of rare diseases. What does this mean? In order for a drug to be classified as an orphan drug, it must treat a disease that affects fewer than 200,000 individuals in the United States or fewer than 5,000 people in the European Union.

Orphan drugs are often extremely expensive, and are generally not covered or reimbursed by insurance companies. It is estimated that there are approximately 7,000 people who die every day because they cannot afford to pay for medication. Some examples of these are cystic fibrosis, hemophilia, muscular dystrophy, leukemia, and AIDS.
Global Orphan Drugs Market Revenue (USD Mn), 2021–2031:

The use of orphan drugs is becoming more common as the global geriatric population figures to increase. These drugs are designed to treat rare diseases and conditions. Pharmaceutical companies have indexed a significant amount of success with these drugs due to their ability to be higher priced than traditional medicines. An increase in the number of patients has been accompanied by a spike in the demand for orphan drugs. These drugs tend to be high-cost pharmaceutical products with small profit margins. The number of orphan drug approvals has increased in recent years with the introduction of new therapeutic approaches and a better understanding of rare diseases.
Orphan drugs are special formulations of drugs that are used to treat rare diseases. Individuals who suffer from these conditions sometimes have no other treatment options, making orphan drug treatments a particularly important form of medicine. The use of these drugs has increased over the past two decades, with the number of new orphan drugs increasing by 60% since 2000. Moreover, government authorities in numerous nations throughout the world are pushing the research and marketing of these orphan drugs. As a result, the orphan medicine industry is likely to expand. These are certain factors that are anticipated to augment this market further in the years to come.
The orphan drugs market is one of the smallest of all FDA-approved drug product markets, with only 13 orphan drugs approved in 2017. This may be accredited to the high cost of these drugs which makes them an unaffordable option for many patients, additionally, it can be difficult to find a customer base for such a small group of people. Furthermore, pharmaceutical companies may not think that developing an orphan drug is worth the effort because they are small in number and require significant research & development investments.
Orphan drugs are medications that are not marketed for mass usage. These types of medicines are only designed to treat a small number of people because there is no clinical necessity to treat the masses. Due to their lack of availability, these drugs are often misunderstood and misused. Moreover, these expensive medications are not covered by most insurance companies and may only be available through patient assistance programs. Factors such as these are anticipated to negatively impact this market’s growth trajectory over the next decade.
The FDA has created a new fast-track pathway for orphan drug development to incentivize innovation in this area. In addition, the FDA has been working to shorten the time from drug discovery to patient consumption. When examining the current state of pharmaceutical research, it is clear that orphan drugs represent a potentially lucrative opportunity for biomedical companies. This factor is expected to be beneficial for existing as well as emerging players in the global orphan drugs market.
Chiasma Inc. reported in June 2020 that their product offering of Mycapssa for the treatment of acromegaly, a rare illness, had received FDA approval.
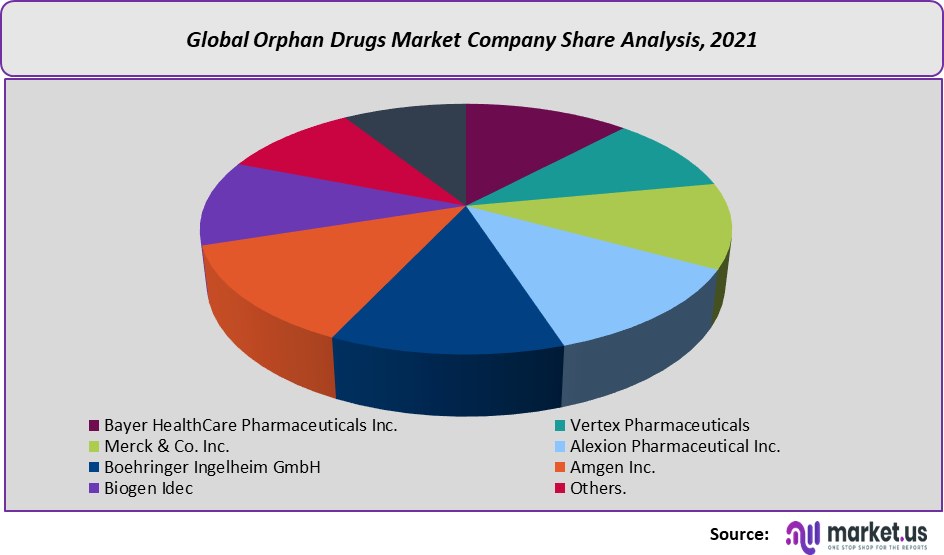
This research report on the global orphan drugs market includes major company profiles such as
- Bayer HealthCare Pharmaceuticals Inc.
- Vertex Pharmaceuticals
- Merck & Co. Inc.
- Alexion Pharmaceutical Inc.
- Boehringer Ingelheim GmbH
- Amgen Inc.
- Biogen Idec
- Actelion Pharmaceuticals Ltd.
- AstraZeneca Plc
- Janssen Biotech Inc.
- Others.
Global Orphan Drugs Market Segmentation is Based on Disease Types, Indications, Distribution Channels, and Region
Based on Disease Types
- Oncologic Diseases
- Metabolic Diseases
- Hematologic and Immunologic Diseases
- Infectious Diseases
- Neurologic Diseases
- Other Rare Diseases
Based on Indications
- Non-Hodgkin Lymphoma
- Acute Myeloid Leukemia
- Cystic Fibrosis
- Glioma
- Pancreatic Cancer
- Ovarian Cancer
- Multiple Myeloma
- Duchenne Muscular Dystrophy
- Graft vs Host Disease
- Renal Cell Carcinoma
- Other Indications
By Distribution Channels
- Hospital Pharmacies
- Online Pharmacies
- Retail Pharmacies
- Other Distribution Channels
Based on Region
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
For the Orphan Drugs Market Market research study, the following years have been considered to estimate the market size:
Attribute Report Details Historical Years
2016-2020
Base Year
2021
Estimated Year
2022
Short Term Projection Year
2028
Projected Year
2023
Long Term Projection Year
2032
Report Coverage
Competitive Landscape, Revenue analysis, Company Share Analysis, Manufacturers Analysis, Volume by Manufacturers, Key Segments, Key company analysis, Market Trends, Distribution Channel, Market Dynamics, COVID-19 Impact Analysis, strategy for existing players to grab maximum market share, and more.
Regional Scope
North America, Europe, Asia-Pacific, South America, Middle East & Africa
Country Scope
United States, Canada and Mexico, Germany, France, UK, Russia and Italy, China, Japan, Korea, India and Southeast Asia, Brazil, Argentina, Colombia etc.Saudi Arabia, UAE, Egypt, Nigeria and South Africa
![Orphan Drugs Market Orphan Drugs Market]()
- Bayer HealthCare Pharmaceuticals Inc.
- Vertex Pharmaceuticals
- Merck & Co. Inc.
- Alexion Pharmaceutical Inc.
- Boehringer Ingelheim GmbH
- Amgen Inc.
- Biogen Idec
- Actelion Pharmaceuticals Ltd.
- AstraZeneca Plc Company Profile
- Janssen Biotech Inc.
- Others.
- settingsSettings
Our Clients
|
Single User
$5,999
$2,999
USD / per unit
save 50% |
Multi User
$7,999
$3,499
USD / per unit
save 55% |
Corporate User
$12,999
$4,499
USD / per unit
save 65% | |
|---|---|---|---|
| e-Access | |||
| Data Set (Excel) | |||
| Company Profile Library Access | |||
| Interactive Dashboard | |||
| Free Custumization | No | up to 10 hrs work | up to 30 hrs work |
| Accessibility | 1 User | 2-5 User | Unlimited |
| Analyst Support | up to 20 hrs | up to 40 hrs | up to 50 hrs |
| Benefit | Up to 20% off on next purchase | Up to 25% off on next purchase | Up to 30% off on next purchase |
| Buy Now ($ 2,999) | Buy Now ($ 3,499) | Buy Now ($ 4,499) |