Global Real World Evidence (RWE) Solutions Market Based on Component: (Services, Data Sets, Clinical Setting Data) Based on Therapeutic Area: (Oncology, Cardiovascular, Other Therapeutic Area) Based on End-Users: (Healthcare Providers, Healthcare Payers, Other End Users) by Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2022-2032
- Published date: Feb 2022
- Report ID: 83875
- Number of Pages: 255
- Format:
- keyboard_arrow_up
Real World Evidence (RWE) Solutions Market Overview:
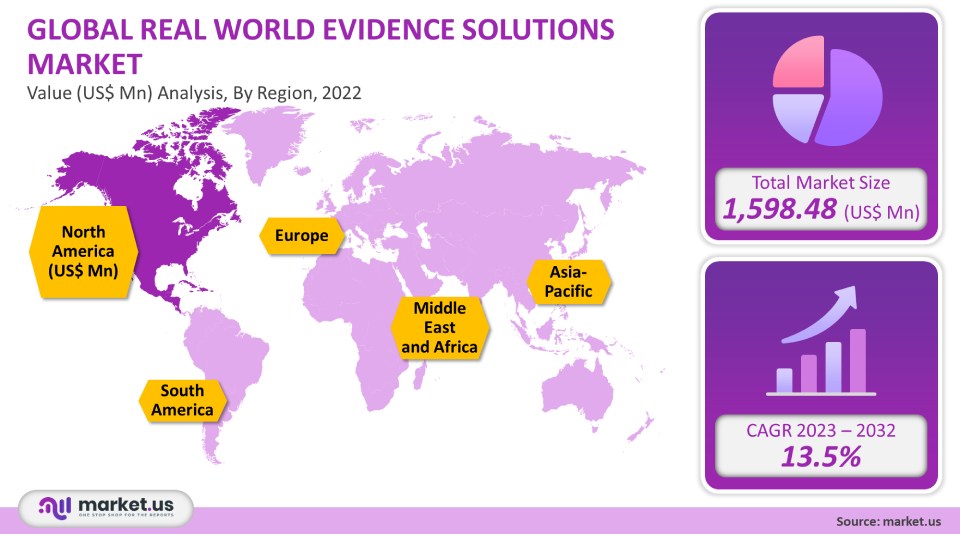
The global real-world evidence/RWE solutions market is projected to reach a valuation of USD 5,671.07 Mn by 2032 at a CAGR of 13.5%, from USD 1,598.48 Mn in 2022.
Every day, new devices and devices with new features are launched. The plethora of Real World Evidence (RWE) that has been collected to help us make informed decisions on these devices has become essential.

Real-world evidence or RWE is the use of data collected from large groups of patients in real-world settings to support medical decision-making. The “real world” refers to all areas outside a traditional clinical trial setting which includes different patient populations, treatments, and dosing schedules. RWE is increasingly being used as an effective tool to generate evidence for healthcare stakeholders to support treatment decisions.
With the growing number of patients and limited medical personnel, the cost of healthcare is continually on the rise. In order to stay competitive in this market, healthcare providers must find new ways to keep costs down and work towards more efficient ways of reaching their goals. Real-world evidence solutions help hospitals receive insights on how to improve processes in order to decrease personnel hours spent on routine tasks, which in turn decreases overall costs.
Global Real World Evidence/RWE Solutions Market Revenue (USD Mn), 2021–2031:

Traditional pharmacovigilance tools (such as the Periodic Benefit-Risk Evaluation Report, Periodic Safety Update Report, and Vaccine Adverse Event Reporting System) as well as newer digital tools, such as the FDA Sentinel Initiative, a post-market active safety surveillance system, are used by regulators to monitor the safety of marketed products. RWE is presently being utilized in pre-approved efficacy decisions, and it has the potential to be used more widely in oncology, to treat uncommon diseases, and for pediatric ailments where randomized controlled clinical trials are impractical or unethical to execute.
Legislators are also understanding the importance of RWE. The United States’ 21st Century Cures Act, passed in December 2016, established public-private partnerships to collect data and increase illness understanding. It also promotes patient-centered medication research and has updated the design and review of clinical studies. Regulators are also expressing a wish to make RWE a significantly bigger part of their operations. This is reflected in the FDA’s efforts to build a single system to monitor the safety of medical products by combining data from electronic medical records, claims data, and registries.
Similarly, the National Institutes of Health (NIH) established a common fund to develop infrastructure, operational knowledge, and capacity for “pragmatic research,” which integrates electronic health records and other real-world data into large-scale distributed research networks to allow researchers to more easily identify a network of interest and speed-up studies.
A fundamental difficulty in this sector is the lack of globally acknowledged rules or principles for the design, conduct, analysis, and reporting of RWE. Because of this lack of agreement, RWE is frequently not considered of adequate quality to be included in the body of data used to compare the efficacy of various treatment choices. This reduces the incentive to generate information by lowering the potential value of the information produced. Furthermore, important players are hesitant to adopt RWE as a result of this.
The healthcare ecosystem is always evolving. Globally, “value” is being scrutinized more closely as healthcare finance players look for innovative ways to deal with the burden of unsustainable costs and low ROI or return on investment. Companies require robust evidence lifecycle management capability to prove value. This has opened the door for an end-to-end strategy to leverage a life sciences organization’s data, evidence, and knowledge assets, breaking down traditional silos and enabling insight-driven decision-making from R&D to product commercialization. Establishing an effective governance plan, using technologies like cloud and self-service analytics, and offering the capacity to combine data sets and comprehend relevant resources for the required analytics are all part of this (and tactical issues around data access and quality).
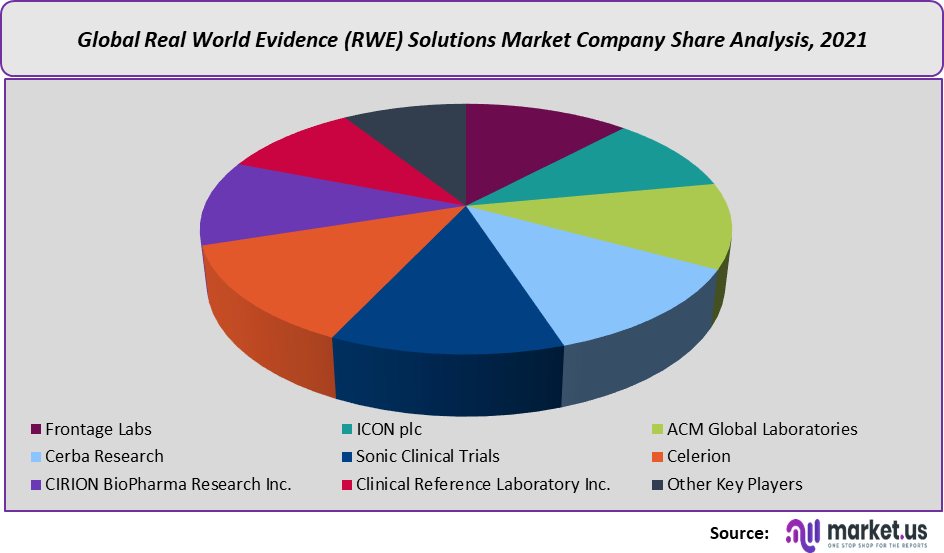
Frontage Labs, ICON Plc, and ACM Global Laboratories are currently offering end-to-end RWE and late-phase services including research planning, protocol preparation, clinical study management, and reporting. The increased need for complete evidence services throughout the product lifecycle is likely to give RWE vendors the opportunity to boost their investments through the entire drug development cycle.

This research report on the global real-world evidence/RWE solutions market includes major company profiles such as:
- Frontage Labs
- ICON plc
- ACM Global Laboratories
- Cerba Research
- Sonic Clinical Trials
- Celerion
- CIRION BioPharma Research Inc.
- Clinical Reference Laboratory Inc.
- Labcorp Drug Development
- Eurofins Scientific
- Others
The Global Real World Evidence/RWE Solutions Market Segmentation is Based on Component, Therapeutic Area, End-User, and Region
Based on Component
- Services
- Data Sets
- Clinical Setting Data
- Claims Data
- Pharmacy Data
- Patient Powered Data
Based on Therapeutic Area
- Oncology
- Cardiovascular
- Neurology
- Immunology
- Other Therapeutic Area
Based on End-Users
- Pharmaceutical and Medical Devices Companies
- Healthcare Providers
- Healthcare Payers
- Other End Users
Based on Region
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
For the Real World Evidence Solutions Market research study, the following years have been considered to estimate the market size:
Attribute Report Details Historical Years
2016-2020
Base Year
2021
Estimated Year
2022
Short Term Projection Year
2028
Projected Year
2023
Long Term Projection Year
2032
Report Coverage
Competitive Landscape, Revenue analysis, Company Share Analysis, Manufacturers Analysis, Volume by Manufacturers, Key Segments, Key company analysis, Market Trends, Distribution Channel, Market Dynamics, COVID-19 Impact Analysis, strategy for existing players to grab maximum market share, and more.
Regional Scope
North America, Europe, Asia-Pacific, South America, Middle East & Africa
Country Scope
United States, Canada and Mexico, Germany, France, UK, Russia and Italy, China, Japan, Korea, India and Southeast Asia, Brazil, Argentina, Colombia etc.Saudi Arabia, UAE, Egypt, Nigeria and South Africa
![Real World Evidence (RWE) Solutions Market Real World Evidence (RWE) Solutions Market]() Real World Evidence (RWE) Solutions MarketPublished date: Feb 2022add_shopping_cartBuy Now get_appDownload Sample
Real World Evidence (RWE) Solutions MarketPublished date: Feb 2022add_shopping_cartBuy Now get_appDownload Sample - Frontage Labs
- ICON plc
- ACM Global Laboratories
- Cerba Research
- Sonic Clinical Trials
- Celerion
- CIRION BioPharma Research Inc.
- Clinical Reference Laboratory Inc.
- Labcorp Drug Development
- Eurofins Scientific
- Others
- settingsSettings
Our Clients
|
Single User
$5,999
$2,999
USD / per unit
save 50% |
Multi User
$7,999
$3,499
USD / per unit
save 55% |
Corporate User
$12,999
$4,499
USD / per unit
save 65% | |
|---|---|---|---|
| e-Access | |||
| Data Set (Excel) | |||
| Company Profile Library Access | |||
| Interactive Dashboard | |||
| Free Custumization | No | up to 10 hrs work | up to 30 hrs work |
| Accessibility | 1 User | 2-5 User | Unlimited |
| Analyst Support | up to 20 hrs | up to 40 hrs | up to 50 hrs |
| Benefit | Up to 20% off on next purchase | Up to 25% off on next purchase | Up to 30% off on next purchase |
| Buy Now ($ 2,999) | Buy Now ($ 3,499) | Buy Now ($ 4,499) |