Global Pressure Ulcer Diagnostics Market By Diagnostic (Imaging Techniques, Microbiological Tests, Other Diagnostic Tests), By End-User (Hospitals, Nursing Homes, Homecare Settings, Other End-Users), By Region, and Key Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2021-2031
- Published date: Jan 2022
- Report ID: 74475
- Number of Pages: 347
- Format:
- keyboard_arrow_up
Pressure ulcers, commonly known as bedsores or pressure sores, are skin and tissue lesions produced mostly by continuous pressure on the skin. Pressure ulcers range in severity from discolored patches to open lesions exposing the underlying bone or muscle.
Heels, elbows, hips, and the base of the spine are frequent sites for pressure ulcers. Individuals with mobility issues are more likely to develop pressure ulcers. Due to variables such as reduced aging of the skin, blood flow. Elderly individuals face a greater proportion of mobility problems, with people over the age of 70 being particularly sensitive to pressure ulcers.
Primary driving factors in the pressure ulcer treatment market are technical developments in wound care therapy and an increase in geriatric populations.

Detailed Segmentation –
Based on Diagnostic Test:
- Imaging Techniques
- Microbiological Tests
- Other Diagnostic Tests
Based on End-User:
- Hospitals
- Nursing Homes
- Homecare Settings
- Other End-Users
Based on Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Market Dynamics –
Drivers for the Global Pressure Ulcer Diagnostics Market:
A significant increase in the senior population living with impairments, a quickly surging prevalence of pressure ulcers, and the increased acceptance of new wound care devices for their treatment are factors expected to augment this market. Owing to surging awareness about the availability of treatment options for pressure ulcers, an increase in healthcare expenditures, as well as improved treatment processes, are expected to develop the pressure ulcer treatment market over the forecast period.
Additionally, an increase in the desire for multiple treatment options by patients diagnosed globally is expected to boost the pressure ulcer market’s overall size.
Restraints Global Pressure Ulcer Diagnostics Market:
High treatment costs and adverse reimbursement rules are expected to potentially stymie this market’s expansion and pose new challenges to the pressure ulcer diagnostics market.
Opportunities for the Global Pressure Ulcer Diagnostics Market:
A surge in the number of patients with pressure ulcers, booming geriatric populations, an increase in the adoption of artificial skin substitutes, increasing healthcare expenditures, improved government funding, as well as public-private-sector initiatives to raise awareness about the negative impacts of pressure ulcers on a patient’s health and quality of life are factors anticipated to lead to the expansion of the pressure ulcer treatment market.
The pressure ulcer treatment market will benefit from increased research & development activities, high unmet demands, as well as the development of treatment and diagnostic technologies.
Trends for the Global Pressure Ulcer Diagnostics Market:
With the development of innovative technologies for the diagnosis and management of illnesses, technological advancements are expected to generate a paradigm shift in the global pressure ulcer diagnostics market. Fluorescence imaging techniques used at the point of treatment are widely used and popular to determine the location and presence of bacteria in afflicted areas. This imaging approach is sensitive and precise in determining the number of bacteria in a wound.
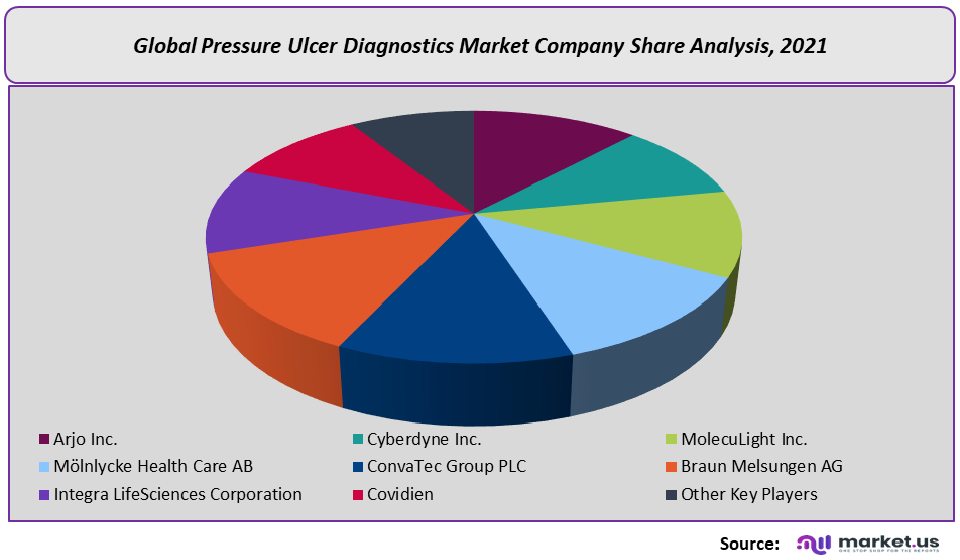
Competitive Landscape –
- Arjo Inc.
- Cyberdyne Inc.
- MolecuLight Inc.
- Mölnlycke Health Care AB
- ConvaTec Group PLC
- Braun Melsungen AG
- Integra LifeSciences Corporation
- Covidien
- IR MED
- Bruin Biometrics LLC.

Recent Developments of Major Players –
- In May 2021, RCSI (Royal College of Surgeons in Ireland) and Bruin Biometrics’ collaborative relationship to innovate the pressure ulcer prevention portfolio was renewed. Bruin Biometrics’ pressure ulcer prevention portfolio will be expanded as a result of this agreement.
- In July, 2021, Esaote SPA installed its new G-scan Brio machine at Istituto Oncologico del Mediterraneo in Viagrande, Catania, Sicily (IOM). This is a novel strategy that would improve the product’s diagnostic capability for a variety of situations.
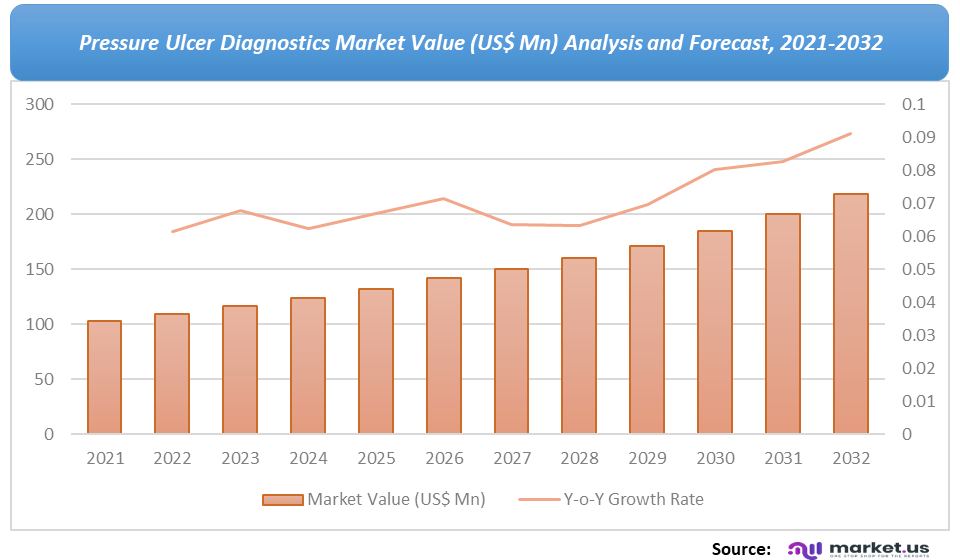
For the Pressure Ulcer Diagnostics Market research study, the following years have been considered to estimate the market size:
Attribute Report Details Historical Years
2016-2020
Base Year
2021
Estimated Year
2022
Short Term Projection Year
2028
Projected Year
2023
Long Term Projection Year
2032
Report Coverage
Competitive Landscape, Revenue analysis, Company Share Analysis, Manufacturers Analysis, Volume by Manufacturers, Key Segments, Key company analysis, Market Trends, Distribution Channel, Market Dynamics, COVID-19 Impact Analysis, strategy for existing players to grab maximum market share, and more.
Regional Scope
North America, Europe, Asia-Pacific, South America, Middle East & Africa
Country Scope
United States, Canada and Mexico, Germany, France, UK, Russia and Italy, China, Japan, Korea, India and Southeast Asia, Brazil, Argentina, Colombia etc.Saudi Arabia, UAE, Egypt, Nigeria and South Africa
![Pressure Ulcer Diagnostics Market Pressure Ulcer Diagnostics Market]() Pressure Ulcer Diagnostics MarketPublished date: Jan 2022add_shopping_cartBuy Now get_appDownload Sample
Pressure Ulcer Diagnostics MarketPublished date: Jan 2022add_shopping_cartBuy Now get_appDownload Sample - Arjo Inc.
- Cyberdyne Inc.
- MolecuLight Inc.
- Mölnlycke Health Care AB
- ConvaTec Group PLC
- B. Braun Melsungen AG Company Profile
- Integra LifeSciences Corporation
- Covidien
- IR MED
- Bruin Biometrics LLC.
- settingsSettings
Our Clients
|
Single User
$5,999
$2,999
USD / per unit
save 50% |
Multi User
$7,999
$3,499
USD / per unit
save 55% |
Corporate User
$12,999
$4,499
USD / per unit
save 65% | |
|---|---|---|---|
| e-Access | |||
| Data Set (Excel) | |||
| Company Profile Library Access | |||
| Interactive Dashboard | |||
| Free Custumization | No | up to 10 hrs work | up to 30 hrs work |
| Accessibility | 1 User | 2-5 User | Unlimited |
| Analyst Support | up to 20 hrs | up to 40 hrs | up to 50 hrs |
| Benefit | Up to 20% off on next purchase | Up to 25% off on next purchase | Up to 30% off on next purchase |
| Buy Now ($ 2,999) | Buy Now ($ 3,499) | Buy Now ($ 4,499) |